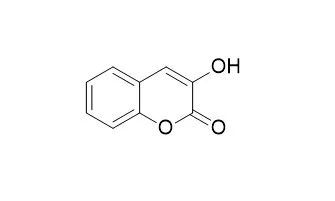
| In vitro: |
| Biochemical & Biophysical Research Communications, 1990, 168(1):169-175. | | 3-Hydroxycoumarins: First direct preparation from coumarins using a Cu2+-ascorbic acid-O2 system, and their potent bioactivities.[Reference: WebLink] |
METHODS AND RESULTS:
First direct 3-hydroxylation of a coumarin ring via a purely chemical system, previously only possible using cytochrome P-450, was successfully conducted by a Cu 2+-ascorbic acid-O 2 system; the two 3-Hydroxycoumarins obtained were novel compounds, 3-hydroxyscopoletin and 3-hydroxyisoscopoletin. 5-Lipoxygenase and α-D-glucosidase inhibitory activities of coumarins greatly increased through 3-hydroxylation.
CONCLUSIONS:
3-Hydroxyscopoletin and 3-hydroxyumbelliferone had a high inhibitory potency for 5-lipoxygenase and for α-D-glucosidase respectively; they serve as lead compounds for new drugs. | | Colloids & Surfaces B Biointerfaces, 2018, 171:675-681. | | 3-Hydroxycoumarin Loaded Vesicles for Recombinant Human Tyrosinase Inhibition in Topical Applications.[Reference: WebLink] | Tyrosinase is one of the key enzymes in mammalian melanin biosynthesis. Decreasing tyrosinase activity has been targeted for the prevention of conditions related to the hyperpigmentation of the skin, such as melasma and age spots. This paper is devoted to the engineering of vesicle formulations loaded with 3-Hydroxycoumarin for topical pharmaceutical applications.
METHODS AND RESULTS:
At first, it was demonstrated the strong inhibiting ability of
3-Hydroxycoumarin against recombinant human tyrosinase. Then, such a drug was effectively encapsulated within liquid or gel-like vesicle formulations, both based on monoolein and lauroylcholine chloride. In vitro skin penetration and permeation studies proved these formulations efficiently overcome the barrier represented by the stratum corneum, delivering 3-Hydroxycoumarin to the deeper skin layers. The effect of applying for different times the liquid and the gel formulation was also evaluated. Results revealed that application of the gel formulation for 2 h favored the drug accumulation into the skin with low transdermal delivery, thus indicating this combination of administration time and formulation as ideal to locally inhibit tyrosinase activity with minimal systemic absorption. Moreover, when incubated with B16F10 melanoma cells, the liquid vesicle formulations did not show cytotoxic activity. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)