| Structure Identification: |
| Biologia Plantarum, 1970, 12(3):246-255. | | Pathways of IAA production from tryptophan by plants and by their epiphytic bacteria: Metabolism of indolepyruvic acid and indolelactic acid.[Reference: WebLink] |
METHODS AND RESULTS:
Metabolites of indolepyruvic acid and Indolelactic acid were investigated using 2 systems: a bacterial (pea stem homogenates containing the epiphytic bacteria) and a plant system (pea stem sections under sterile conditions). The products of spontaneous indolepyruvic acid decomposition in aqueous solution and during chromatography were investigated, too.Biological indolepyruvic acid conversion yields, besides those substance amounts which occur spontaneously, indoleacetic acid, indoleethanol (tryptophol) and (only in the sterile plant system) indoleacetaldehyde. An inhibitor extract from pea stems decreases the indoleacetic acid and increases the indoleethanol and indoleacetaldehyde gain.
CONCLUSIONS:
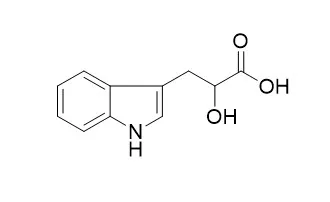
Indolelactic acid is not metabolized in the sterile plant sections. Indolelactic acid oxidation by the bacteria-containing homogenate yields indolepyruvic acid and is inhibited by the inhibitor extract. | | Journal of Chromatography A, 2015, 205( 1):125-137. | | Identification of 3-indoleacetic acid in pinus sylvestris L. by gas chromatography-mas spectrometry, and quantitative analysis by ion-pair reversed-phase liquid chromatography wit spectrofluorimetric detection.[Reference: WebLink] |
METHODS AND RESULTS:
The phytochormone 3-indoleacetic acid (IAA) was conclusively identified by gas chromatography-mas spetrometry of its methyl ester in purified extracts of Scots pine, Pinus sylvestris L. Preceding purification included reversed-phase ion-pair chromatography on a Nucleosil C18 column with tetrabutylammonium ion as counter ion. A number of indole acids were separated in this high-performance liquid chromatographic (HPLC) system. In particular, IAA was separated from the reported plant constituents 4-chloro-IAA, 5-hydroxy-IAA, 3-indoleacrylic acid, 3-Indolelactic acid, 3-indolepropionic acid, 3-indolebutyric acid and tytophan. In connection with a spectrofluorine detector, the HPLC system was also used for the quantitative analysis of IAA in pine samples. Systematic errors caused a constant under-estimation of the IAA content by 12% and could easily be corrected for. The random error was 14%. Eight-week-old pine seedings contained 46 ng g−1 of IAA.
CONCLUSIONS:
The sensitivity of the method, applied to the analysis of pine extracts, was 50 pg of IAA. Evidence is presented that the method, when applied to the analysis of pine extracts, is specific of IAA. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)