| Kinase Assay: |
| Phytomedicine. 2014 Sep 25;21(11):1303-9. | | Prenylated xanthones from mangosteen as promising cholinesterase inhibitors and their molecular docking studies.[Pubmed: 25172794] | Garcinia mangostana is a well-known tropical plant found mostly in South East Asia. The present study investigated acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activities of G. mangostana extract and its chemical constituents using Ellman's colorimetric method.
METHODS AND RESULTS:
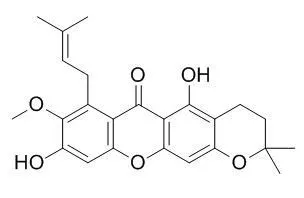
Cholinesterase inhibitory-guided approach led to identification of six bioactive prenylated xanthones showing moderate to potent cholinesterases inhibition with IC50 values of lower than 20.5 μM. The most potent inhibitor of AChE was garcinone C while γ-mangostin was the most potent inhibitor of BChE with IC50 values of 1.24 and 1.78 μM, respectively. Among the xanthones, mangostanol, 3-Isomangostin, garcinone C and α-mangostin are AChE selective inhibitors, 8-deoxygartanin is a BChE selective inhibitor while γ-mangostin is a dual inhibitor. Preliminary structure-activity relationship suggests the importance of the C-8 prenyl and C-7 hydroxy groups for good AChE and BChE inhibitory activities. The enzyme kinetic studies indicate that both α-mangostin and garcinone C are mixed-mode inhibitors, while γ-mangostin is a non-competitive inhibitor of AChE. In contrast, both γ-mangostin and garcinone C are uncompetitive inhibitors, while α-mangostin is a mixed-mode inhibitor of BChE. Molecular docking studies revealed that α-mangostin, γ-mangostin and garcinone C interacts differently with the five important regions of AChE and BChE.
CONCLUSIONS:
The nature of protein-ligand interactions is mainly hydrophobic and hydrogen bonding. These bioactive prenylated xanthones are worthy for further investigations. | | Phytomedicine. 2015 Jan 15;22(1):49-51. | | The inhibitory activity of aldose reductase in vitro by constituents of Garcinia mangostana Linn.[Pubmed: 25636870] |
METHODS AND RESULTS:
We investigated aldose reductase inhibition of Garcinia mangostana Linn. from Indonesia. Dichloromethane extract of the root bark of this tree was found to demonstrate an IC50 value of 11.98 µg/ml for human aldose reductase in vitro. From the dichloromethane fraction, prenylated xanthones were isolated as potent human aldose reductase inhibitors.
CONCLUSIONS:
We discovered 3-Isomangostin to be most potent against aldose reductase, with an IC50 of 3.48 µM. | | Food Chem Toxicol. 2013 Sep;59:793-800. | | Inhibitory effects of α-mangostin on mammalian DNA polymerase, topoisomerase, and human cancer cell proliferation.[Pubmed: 23811100] | We found that the ethanol extract of mangosteen (Garcinia mangostana L.) fruit rind had a strong inhibitory effect on mammalian DNA polymerase (pol) activity and isolated α-mangostin as a potent pol inhibitor from the extract.
METHODS AND RESULTS:
In this study, the inhibitory activities against mammalian pols by α-mangostin and its related five compounds, 3-Isomangostin, xanthone, 9,10-anthraquinone, 9-anthracenecarboxylic acid, and anthracene, were investigated. α-Mangostin was the most potent inhibitor of the mammalian pol species among the tested compounds, with IC₅₀ values of 14.8-25.6 μM. This compound also inhibited human DNA topoisomerases (topos) I and II activities with IC₅₀ values of 15.0 and 7.5 μM, respectively, but did not inhibit the activities of other DNA metabolic enzymes tested. α-Mangostin also did not directly bind to double-stranded DNA as determined by thermal transition analysis. α-Mangostin was found to suppress human colon HCT116 carcinoma cell proliferation with an LC₅₀ of 18.5 μM, inhibit the activity of cellular topos, halt cell cycle in the G2/M phase, and induce apoptosis.
CONCLUSIONS:
These results suggest that decreased proliferation by α-mangostin may be a result of the inhibition of cellular topos rather than pols, and α-mangostin might be an anticancer chemotherapeutic agent. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)