| In vitro: |
| Tetrahedron, 2002, 58(40):8073-8086. | | Cytotoxic and anti-HIV-1 constituents of Gardenia obtusifolia and their modified compounds.[Reference: WebLink] |
METHODS AND RESULTS:
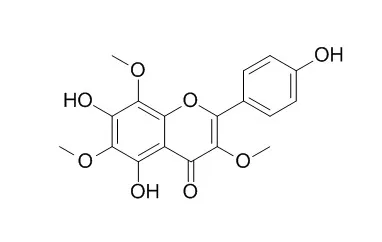
5α-Cycloart-24-ene-3,23-dione (1), 5α-cycloart-24-ene-3,16,23-trione (2) and methyl 3,4-seco-cycloart-4(28),24-diene-29-hydroxy-23-oxo-3-oate (3), together with five known flavones 5,7,4′-trihydroxy-3,8-dimethoxyflavone (4), 5,7,4′-trihydroxy-3,8,3′-tri-methoxyflavone (5), 5,7,4′-trihydroxy-3,6,8-trimethoxyflavone (4',5,7-Trihydroxy 3,6,8-trimethoxyflavone,6), 5,4′-dihydroxy-3,6,7,8-tetramethoxyflavone (7) and 5,3′-dihydroxy-3,6,7,8,4′-pentamethoxyflavone (8) have been isolated from the leaves and twigs of Gardenia obtusifolia. The structures were assigned on the basis of spectroscopic methods.
CONCLUSIONS:
Compounds 3–8 and some of the modified compounds showed significant cytotoxic activities in several mammalian cell lines, especially 8 and its diacetate 21 which exhibited potent cytotoxicities (compound 8: P-388 0.05 μg/mL, KB 0.09 μg/mL, BCA-1 0.63 μg/mL, Lu-1 0.09 μg/mL, ASK 0.70 μg/mL; its diacetate: P-388 0.27 μg/mL, KB 0.06 μg/mL, BCA-1 0.53 μg/mL, Lu-1 0.49 μg/mL). It was also found that 5, 8 and 21 showed antimitotic acitivity in the ASK assay. Compounds 2, 4, 6, 7 and some of the modified compounds displayed interesting anti-HIV activity in the syncytium assay, but were inactive or exhibited weak activity in the HIV-1 RT assay; while compound 3 was found to be active in the HIV-1 RT assay (99.9 % inhibition at 200 μg/mL), but cytotoxic in the syncytium assay.
|
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)