| In vitro: |
| Molecules. 2008 Jun 1;13(6):1219-29. | | Identification of minor secondary metabolites from the latex of Croton lechleri (Muell-Arg) and evaluation of their antioxidant activity.[Pubmed: 18596648] | Dragon's blood (Sangre de drago), a viscous red sap derived from Croton lechleri Muell-Arg (Euphorbiaceae), is extensively used by indigenous cultures of the Amazonian basin for its wound healing properties. The aim of this study was to identify the minor secondary metabolites and test the antioxidant activity of this sustance.
METHODS AND RESULTS:
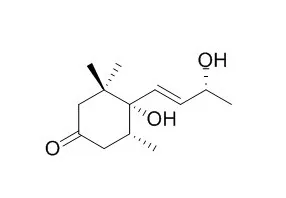
A bioguided fractionation of the n-hexane, chloroform, n-butanol, and aqueous extracts led to the isolation of 15 compounds: three megastigmanes, four flavan-3-ols, three phenylpropanoids, three lignans, a clerodane, and the alkaloid taspine. In addition to these known molecules, six compounds were isolated and identified for the first time in the latex: blumenol B, blumenol C, 4,5-Dihydroblumenol A, erythro-guaiacyl-glyceryl-beta-O-4'- dihydroconiferyl ether, 2-[4-(3-hydroxypropyl)-2-methoxyphenoxy]-propane-1,3-diol and floribundic acid glucoside. Combinations of spectroscopic methods ((1)H-, (13)C- NMR and 2D-NMR experiments), ESI-MS, and literature comparisons were used for compound identification.
In vitro antioxidant activities were assessed by DPPH, total antioxidant capacity and lipid peroxidation assays.
CONCLUSIONS:
Flavan-3-ols derivatives (as major phenolic compounds in the latex) exhibited the highest antioxidant activity. | | Chinese Traditional & Herbal Drugs, 2007, 38(7): 976-9. | | Antivirus constituents from Alternanthera philoxeroides.[Reference: WebLink] | To investigate the anti-HBV constituents from Alternanthera philoxeroides.
METHODS AND RESULTS:
The constituents were isolated with silica gel and gel permeation chromatography,and purified by HPLC.Their structures were elucidated by spectroscopy.The antivirus effects of the isolated compounds were tested by ELISA method in vitro. Ten compounds were isolated and elucidated as followings:oleanolic acid(Ⅰ),oleanolic acid 3-O-β-D-glucuronopyranoside(Ⅱ),oleanolic acid 28-O-β-D-glucopyranoside(Ⅲ),chikusetsusaponin Ⅳ a methyl ester(Ⅳ),4,5-dihydroblumenol(Ⅴ),N-trans-feruloyl 3-methyldopamine(Ⅵ),N-trans-feruloyl tyramine(Ⅶ),3β-hydroxystigmast-5-en-7-one(Ⅷ),24-methylenecycloartanol(Ⅸ),and cycloeucalenol(Ⅹ).The values of inhibition percent of compounds Ⅰ-Ⅲ,Ⅴ-Ⅶ revealed a significant distinction compared to the control group.Compounds Ⅱ and Ⅴ showed significant inhibition against HepG2 cells transected with cloned hepatitis B virus DNA,their inhibitive ratios were 85.38% and 87.37% at 50 μg/mL,respectively.
CONCLUSIONS:
Compounds Ⅳ-Ⅶ are isolated from this plant for the first time and phenolic amides have been determined as the new structure type from the plants of Alternanthera Forsk.Compounds Ⅱ and Ⅴ from A.philoxeroides show the more significant anti-HBV activities. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)