| Structure Identification: |
| Chemistry, 2015, 20(42):13644-13655. | | Synthesis of New Chlorin e6 Trimethyl and Protoporphyrin IX Dimethyl Ester Derivatives and Their Photophysical and Electrochemical Characterizations.[Reference: WebLink] | In view of increasing demands for efficient photosensitizers for photodynamic therapy (PDT), this paper reports the synthesis and photophysical characterizations of new chlorin e6 trimethyl ester and protoporphyrin-IX dimethyl ester dyads as free-bases and Zn(II) complexes.
METHODS AND RESULTS:
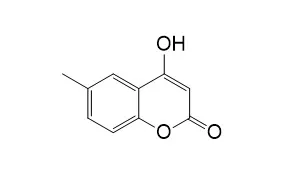
The synthesis of these molecules linked at the β-pyrrolic positions to pyrano[3,2-c]coumarin, pyrano[3,2-c]quinolinone and pyrano[3,2-c]naphthoquinone moieties was performed by using the domino Knoevenagel hetero Diels-Alder reaction. The α-methylenechromanes, α-methylenequinoline and ortho-quinone methides were generated in situ from Knoevenagel reaction respectively of 4-hydroxycoumarin, 4-Hydroxy-6-methylcoumarin, 4-hydroxy-N-methylquinolinone and 2-hydroxy-1,4-naphthoquinone with paraformaldehyde in dioxane. All the dyads as free-bases and as Zn(II) complexes were obtained in high yields. All new compounds were fully characterized by 1D and 2D NMR techniques, UV-Vis and HRMS. Their photophysical properties were evaluated by measuring the fluorescence, singlet oxygen quantum yield by luminescence detection and also the triplet lifetimes were correlated by flash photolysis and intersystem crossing (ISC) rates. The fluorescence lifetimes were measured by time correlated single photon count (TCSPC) method, fluorescence decay associated spectra (FDAS) and anisotropy measurements.
CONCLUSIONS:
Magnetic circular dichroism (MCD) and circular dichroism (CD) spectra were recorded for one Zn(II) complex in order to obtain information, respectively, on the electronic and conformational states, and interpretation of these spectra was enhanced by molecular orbital (MO) calculations. Electrochemical studies of the Zn(II) complexes were also carried out to gain insights into their behavior for such applications. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)