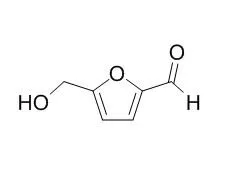
| Structure Identification: |
| J Sep Sci. 2012 Oct;35(19):2567-74. | | Development and validation of an HPLC method to determine metabolites of 5-hydroxymethylfurfural (5-HMF).[Pubmed: 22941583] | The food component 5-Hydroxymethylfurfural is supposed to have antioxidative properties and is therefore used as an acting agent in a novel anticancer infusion solution, named Karal®, and an oral supplementation. Previous studies showed that after oral and intravenous application, the substance is completely decomposed to its metabolites: 5-hydroxymethylfuroic acid, 2,5-furandicarboxylic acid, and N-(hydroxymethyl)furoyl glycine. The formation of a fourth metabolite, namely 5-sulphoxymethylfurfural, is still not clarified according to literature.
METHODS AND RESULTS:
Due to commercial unavailability, synthesis of 5-sulphoxymethylfurfural was conducted and a synthesis procedure for N-(hydroxymethyl)furoyl glycine had to be developed. Identification of the synthesised compounds was proven by LC-MS and NMR. An appropriate HPLC method was established to obtain good separation of the four possible metabolic substances and 5-Hydroxymethylfurfural within 12 min via a HILIC column (150 × 4.6 mm, 5 μm) using a gradient grade system switching from mobile phase A (ACN/ammonium formate 100 mM, pH 2.35, 95:5, v/v) to mobile phase B (ACN/ammonium formate 100 mM, pH 2.35, 85:15, v/v).
CONCLUSIONS:
The procedure was afterward validated following ICH guidelines in terms of selectivity, linearity, precision, LOD, and LOQ. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)