| In vitro: |
| Phytochemistry. 2000 Apr;53(8):833-6. | | Anti-platelet aggregation constituents from Gynura elliptica.[Pubmed: 10820787] |
METHODS AND RESULTS:
A p-hydroxyacetophenone-like derivative, (+)-gynunone, and a chromane, together with six known compounds were isolated from the CHCl3 fraction of the roots of Gynura elliptica.
Their structures were determined by means of spectral analyses.
CONCLUSIONS:
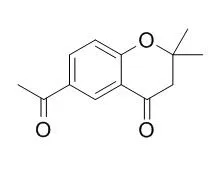
Among the isolates, 6-Acetyl-2,2-dimethylchroman-4-one and vanillin showed anti-platelet aggregation activity induced by arachidonic acid in vitro. | | Chem Biodivers. 2007 Dec;4(12):2835-44. | | Studies on the cytotoxicity of miscellaneous compounds from Eupatorium betonicaeforme (D.C.) Baker (Asteraceae).[Pubmed: 18081094 ] |
METHODS AND RESULTS:
A detailed study on the cytotoxic effects of five known constituents isolated from the flowers and roots of Eupatorium betonicaeforme is reported, including 2,2-dimethyl-6-vinylchroman-4-one (1), 2-senecioyl-4-vinylphenol (2), 6-Acetyl-2,2-dimethylchroman-4-one (3), (4E)-8beta-angeloyloxy-9beta,10beta-dihydroxy-1-oxogermacra-4,11(13)-dien-12,6alpha-olide (4), and 3beta-hydroxyicosan-1,5beta-olide (5). The sesquiterpene lactone 4 exhibited the highest cytotoxicity, with IC50 values ranging from 3.9 to 9.9 microM, showing some degree of cell selectivity. The antiproliferative activity of 4 was examined towards HL-60 cells, and found to diminish cell viability in a dose-dependent manner. Moreover, at all concentrations tested, there was a decrease in the number of cells capable of incorporating 5-bromo-2'-deoxyuridine (BrdU), indicating disruption of DNA synthesis. The morphological changes induced by 4 were compatible with apoptotic cell death.
CONCLUSIONS:
This work, thus, corroborates the anticancer potential of Eupatorium secondary metabolites. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)