| In vitro: |
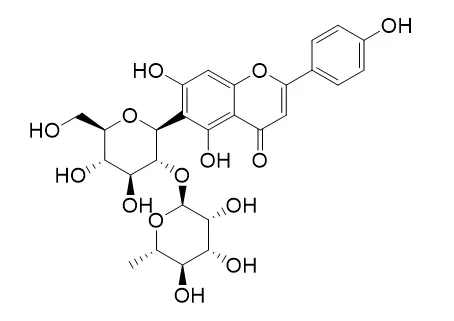
| J Agric Food Chem . 2013 Nov 6;61(44):10507-15. | | Qualitative and quantitative analysis of phenolics in Tetrastigma hemsleyanum and their antioxidant and antiproliferative activities[Pubmed: 24151872] | | The phenolic profiles of Tetrastigma hemsleyanum leaf extracts by different solvents (80% methanol, ethyl acetate and hexane) and their antioxidant and antiproliferative activities were investigated. Thirteen phenolic compounds (3-caffeoylquinic acid, 5-caffeoylquinic acid, 1-caffeoylquinic acid, 5-p-coumaroylquinic acid, isoorientin-2″-O-rhamnoside, isoorientin, orientin-2″-O-rhamnoside, orientin, 1-p-coumaroylquinic acid, vitexin-2″-O-rhamnoside, isovitexin-2″-O-rhamnoside, vitexin and isovitexin) were identified in T. hemsleyanum leaves for the first time, and six of them were quantified using a combination of LC-QTOF-MS and LC-QqQ-MS techniques. It was found that 80% methanol extract exhibited the highest antioxidant activities (DPPH, 3.32 mmol of Trolox/g DW; ABTS, 1.38 mmol of Trolox/g DW; FRAP, 1.85 mmol of FeSO4/g DW), while the hexane extract had the lowest (1.23, 0.43 and 0.13, respectively). Total phenolic contents (TPC) of various extracts of T. hemsleyanum leaves ranged from 28.95 to 275.71 mg of GAE/g DW. Also, total antioxidant activities as evaluated by ABTS, FRAP and DPPH assays were correlated well with TPC. In addition, 80% methanol extract provided antiproliferative activity on HepG2 cells (IC50 = 524 μg/mL). This paper provides a complete picture of phenolics in T. hemsleyanum leaves and relates them to their antioxidant and antiproliferative activities. | | Planta Med . 1992 Dec;58(6):544-8. | | Antihepatotoxic C-glycosylflavones from the leaves of Allophyllus edulis var. edulis and gracilis[Pubmed: 1484895] | | From the leaves of Allophyllus edulis var. edulis and Allophyllus edulis var. gracilis nine C-glycosylflavones have been isolated and identified as schaftoside (8), vicenin-2 (9), lucenin-2 (10), isovitexin 2"-O-rhamnoside (11), cerarvensin 2"-O-rhamnoside (12), vitexin 2"-O-rhamnoside (13), isoorientin 2"-O-rhamnoside (15), orientin 2"-O-rhamnoside (16) and saponarin (17). In addition, gallic acid (2), the phenol C-glycosides bergenin (3) and 11-O-galloylbergenin (4), three flavonol 3-O-rhamnosides and a new C-glycosylflavone identified as mollupentin 2"-O-rhamnoside (14) were obtained from the leaves of Allophyllus edulis var. edulis. Their structures were elucidated on the basis of chemical and spectral data. For the first time the C-glycosylflavones were found to have remarkable anti-hepatotoxic activities against CCl4 and galactosamine cytotoxicity in primary cultured rat hepatocytes. Structure-activity relationships are discussed. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)