| Description: |
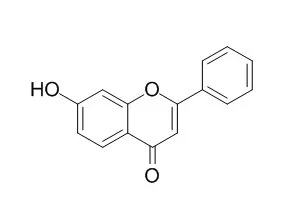
7-Hydroxyflavone is a potent inhibitor of CYP1A1 with a Ki value of 0.015 μM and exhibits 6-fold greater selectivity for CYP1A1 over CYP1A2. It also has excellent antioxidant,
anti-inflammatory, and anti-proliferation properties, it may serve as potential protective agents in the treatment of patients with chronic EV71 infection.7-Hydroxyflavone can protect renal cells from NIC-associated cytotoxicity via the ERK/Nrf2/HO-1 pathway; it also can inhibit LPS-induced inflammation via attenuating the production of NO, PGE2 , TNF-α and IL-6. |
| In vitro: |
| PLoS One. 2014 Mar 24;9(3):e92565. | | Inhibition of Enterovirus 71 replication by 7-hydroxyflavone and diisopropyl-flavon7-yl Phosphate.[Pubmed: 24664133] | Enterovirus 71 (EV71) is the major causative agent of hand, foot, and mouth disease, which has been continuously prevalent in Asia in recent years. In children, severe cases can lead to death, and no prophylactic or therapeutic measures against EV71 infection are available. The 3C proteases of EV71 play an important role in viral replication and are an ideal drug target. In previous work, we resolved the crystal structure for EV71 3Cpro.
METHODS AND RESULTS:
In this report, we took advantage of the automated docking program AutoDock 4.0 to simulate EV71 3Cpro-ligand conformation. 7-Hydroxyflavone (HF) and its phosphate ester(FIP) were predicted to bind with EV71 3Cpro.In an in vitro protease inhibition assay, FIP inhibited EV71 3Cpro protease activity. Both flavones were highly active against EV71, protecting cells from EV71 infection. Replication of viral RNA and formation of EV71 plaque were all strongly inhibited in cells.
CONCLUSIONS:
These results indicated that HF and FIP may serve as potential protective agents in the treatment of patients with chronic EV71 infection. | | PLoS One. 2012;7(5):e36652. | | Inhibitory effects and underlying mechanism of 7-hydroxyflavone phosphate ester in HeLa cells.[Pubmed: 22574207] | Chrysin and its phosphate ester have previously been shown to inhibit cell proliferation and induce apoptosis in Hela cells; however, the underlying mechanism remains to be characterized.
METHODS AND RESULTS:
In the present study, we therefore synthesized diethyl flavon-7-yl phosphate (FP, C(19)H(19)O(6)P) by a simplified Atheron-Todd reaction, and explored its anti-tumor characteristics and mechanisms. Cell proliferation, cell cycle progression and apoptosis were measured by MTS, flow cytometry and terminal deoxynucleotidyl transferase dUTP nick end labeling techniques, respectively in human cervical cancer HeLa cells treated with 7-Hydroxyflavone (HF) and FP. p21, proliferating cell nuclear antigen (PCNA) and cAMP levels in Hela cells were analyzed by western blot and radioimmunoassay. Both HF and FP inhibited proliferation and induced apoptosis in HeLa cells via induction of PCNA/p21 expression, cleaved caspase-3/poly (ADP-ribose) polymerase (PARP)-1, elevation of cAMP levels, and cell cycle arrest with accumulation of cells in the G0/G1 fraction. The effects of FP were more potent than those of HF. The interactions of FP with Ca(2+)-calmodulin (CaM) and Ca(2+)-CaM-phosphodiesterase (PDE)1 were explored by electrospray ionization-mass spectrometry and fluorescence spectra. FP, but not HF, formed non-covalent complexes with Ca(2+)-CaM-PDE1, indicating that FP is an inhibitor of PDE1, and resulting in elevated cellular cAMP levels. It is possible that the elevated cAMP levels inhibit growth and induce apoptosis in Hela cells through induction of p21 and cleaved caspase-3/PARP-1 expression, and causing down-regulation of PCNA and cell cycle arrest with accumulation of cells in the G0/G1 and G2/M fractions. In conclusion, FP was shown to be a Ca(2+)-CaM-PDE inhibitor, which might account for its underlying anti-cancer mechanism in HeLa cells.
CONCLUSIONS:
These observations clearly demonstrate the special roles of phosphorylated flavonoids in biological processes, and suggest that FP might represent a potential new drug for the therapy of human cervical carcinoma. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)