Halimodendron halodendron has been used as forage in northwestern China for a long time. Its young leaves and flowers are edible and favored by indigenous people.
METHODS AND RESULTS:
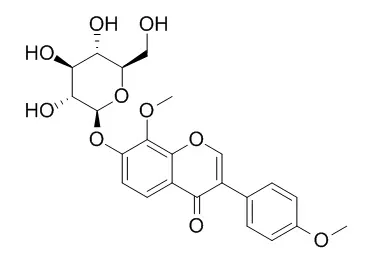
In this study, eleven phenolic compounds were bioassay-guided and isolated from the aerial parts of H. halodendron for the first time. They were identified by means of physicochemical and spectrometric analysis as quercetin (1), 3,5,7,8,4'-pentahydroxy-3'-methoxy flavone (2), 3-O-methylquercetin (3), 3,3'-di-O-methylquercetin (4), 3,3'-di-O-methylquercetin-7-O-β-d-glucopyranoside (5), isorhamentin-3-O-β-d-rutinoside (6), 8-O-methylretusin (7), 8-O-Methylretusin-7-O-beta-D-glucopyranoside (8), salicylic acid (9), p-hydroxybenzoic acid (ferulic acid) (10), and 4-hydroxy-3-methoxy cinnamic acid (11). They were sorted as flavonols (1-6), soflavones (7 and 8), and phenolic acids (9-11). Among the compounds, flanools 1-4 revealed a strong antibacterial activity with minimum inhibitory concentration (MIC) values of 50-150 μg/mL, and median inhibitory concentration (IC(50)) values of 26.8-125.1 μg/mL. The two isoflavones (7 and 8) showed moderate inhibitory activity on the test bacteria. Three phenolic acids (9, 10 and 11) showed strong antibacterial activity with IC(50) values of 28.1-149.7 μg/mL. Antifungal activities of the compounds were similar to their antibacterial activities. All these phenolic compounds showed significant antimicrobial activity with a broad spectrum as well as antioxidant activity based on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging and β-carotene-linoleic acid bleaching assays. In general, the flavonol aglycones with relatively low polarity exhibited stronger activities than the glycosides.
CONCLUSIONS:
The results suggest the potential of this plant as a source of functional food ingredients and provide support data for its utilization as forage as well. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)