| In vitro: |
| J Agric Food Chem. 2009 Aug 26;57(16):7293-7. | | Effects of tea catechins, epigallocatechin, gallocatechin, and gallocatechin gallate, on bone metabolism.[Pubmed: 19653629 ] |
METHODS AND RESULTS:
In this study, three tea catechins, epigallocatechin (EGC), gallocatechin (GC), and (-)-Gallocatechin gallate (GCG), were investigated for their effects on bone metabolism. The effects of the tea catechins on bone formation were evaluated using cultured rat osteoblast-like osteosarcoma cell line UMR-106. EGC stimulated alkaline phosphatase activity significantly at concentrations of 10 and 20 microM. The amount of mineralization also increased significantly with EGC. On another cell culture platform, EGC significantly inhibited osteoclast formations from RAW 264.7 cells upon receptor activation of nuclear factor-kappaB ligand induction on the fourth day of treatment, at a concentration of 10 microM. EGC also dose-dependently inhibited the mRNA expression of tatrate-resistant acid phosphatase. GC and GCG could decrease osteoclastogenesis at 20 microM.
CONCLUSIONS:
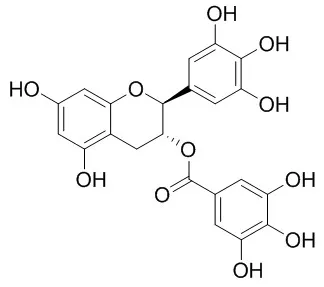
The present study illustrated that the tea catechins, EGC in particular, had positive effects on bone metabolism through a double process of promoting osteoblastic activity and inhibiting osteoclast differentiations. | | J Agric Food Chem. 2003 Dec 3;51(25):7303-7. | | Heat-epimerized tea catechins rich in gallocatechin gallate and catechin gallate are more effective to inhibit cholesterol absorption than tea catechins rich in epigallocatechin gallate and epicatechin gallate.[Pubmed: 14640575] | It has been known that tea catechins, (-)-epicatechin (1), (-)-epigallocatechin (2), (-)-epicatechin gallate (3), and (-)-epigallocatechin gallate (4) are epimerized to(-)-catechin (5), (-)-gallocatechin (6), (-)-catechin gallate (7), and (-)-Gallocatechin gallate (8), respectively, during retort pasteurization. We previously reported that tea catechins, mainly composed of 3 and 4, effectively inhibit cholesterol absorption in rats.
METHODS AND RESULTS:
In this study, the effect of heat-epimerized catechins on cholesterol absorption was compared with tea catechins. Both tea catechins and heat-epimerized catechins lowered lymphatic recovery of cholesterol in rats cannulated in the thoracic duct and epimerized catechins were more effective than tea catechins. The effect of purified catechins on micellar solubility of cholesterol was examined in an in vitro study. The addition of gallate esters of catechins reduced micellar solubility of cholesterol by precipitating cholesterol from bile salt micelles. Compounds 7 and 8 were more effective to precipitate cholesterol than 3 and 4, respectively.
CONCLUSIONS:
These observations strongly suggest that heat-epimerized catechins may be more hypocholesterolemic than tea catechins. | | Food Chem . 2017 Nov 15;235:227-233. | | C-geranylated flavanones from YingDe black tea and their antioxidant and α-glucosidase inhibition activities[Pubmed: 28554631] | | Abstract
YingDe black tea is produced from crude tea prepared from leaves of Camellia sinensis var. assamica. In this work, we isolated and identified five novel flavanones, namely, amelliaone A-E (1-5), along with seven known compounds 6-12 from the ethanol extract of YingDe black tea. The structures of these five novel phenolic compounds were determined using extensive 1D and 2D nuclear magnetic resonance spectroscopy experiments. The compounds were further evaluated for antioxidant, α-glucosidase inhibitory, and cytotoxic activities. Compound 1 exhibited higher α-glucosidase inhibitory activity with a half-maximum inhibitory concentration value (IC50) of 10.2μM compared with acarbose (18.2μM).
Keywords: (−)-Epicatechin (PubChem CID: 72276); (−)-Gallocatechin gallate (PubChem CID: 199472); Amentoflavone (PubChem CID: 5281600); Apigenin (PubChem CID: 5280443); Camellia var. assamica; Epigallocatechin gallate (PubChem CID: 65064); Flavanone; Gallic acid (PubChem CID: 370); Quercetin (PubChem CID: 5280343); YingDe black tea; α-Glucosidase inhibitory activity. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)