| Kinase Assay: |
| Phytomedicine. 2016 Jul 15;23(8):846-55. | | Agrimonolide from Agrimonia pilosa suppresses inflammatory responses through down-regulation of COX-2/iNOS and inactivation of NF-κB in lipopolysaccharide-stimulated macrophages.[Pubmed: 27288920 ] | Agrimonolide from Agrimonia pilosa showed a strong anti-inflammatory activity, and the present study aims to reveal potential mechanisms on molecular level explaining its anti-inflammatory effect.To investigate the mechanism of anti-inflammatory activity of Agrimonolide. Anti-inflammatory activity of Agrimonolide in cells was applied.
METHODS AND RESULTS:
Anti-inflammatory activity of Agrimonolide isolated from Agrimonia pilosa was evaluated using lipopolysaccharide (LPS) stimulated RAW 264.7 cell models. The productions of IL-1β, IL-6, TNF-α and NO were determined by ELISA and nitrite analysis, respectively. The expressions of iNOS and COX-2 were measured by western blotting and RT-PCR analysis.
The pre-treatment with Agrimonolide significantly reduced the levels of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α), as well as attenuated the expression of iNOS and COX-2 in LPS-stimulated macrophages. Furthermore, Agrimonolide inhibited the activation of JNK and p38 MAPKs and decreased the activation of JAK-STAT and NF-κB in LPS-stimulated macrophages.
CONCLUSIONS:
The present study suggested that Agrimonolide exerted anti- inflammatory activity, at least in part, via suppressing LPS-induced activation of JAK-STATs and p38 MAPKs signaling pathway. |
|
| Structure Identification: |
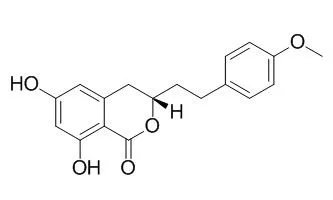
| J Sep Sci. 2012 Aug;35(15):1977-84. | | Preparative purification of five bioactive components from Agrimonia pilosa Ledeb by high-speed counter-current chromatography.[Pubmed: 22865760] |
METHODS AND RESULTS:
High-speed counter-current chromatography (HSCCC) coupled with ultraviolet (UV) detection or evaporative light-scattering detection was successfully applied for preparative separation of five bioactive compounds from Agrimonia pilosa Ledeb. In preliminary process, D101 macroporous resin was used to separate the crude extract of the plant and four fractions (20, 40, 50, and 60% aqueous ethanol elutions) were produced. Then, these fractions were directly subjected to HSCCC purification. Five chemicals including taxifolin-3-glucoside (6.4 mg), quercetin-3-rhamnoside (13.0 mg), tiliroside (14.7 mg), Agrimonolide (21.4 mg), and tormentic acid (29.8 mg) with the purities of 94.24, 95.37, 97.42, 95.29, and 96.34% were separated from each 200 mg prepared fraction. The purities were analyzed by high-performance liquid chromatography, and the chemical structures of the products were identified by UV detection, mass spectrometry, nuclear magnetic resonance, and the standards.
CONCLUSIONS:
This paper used a simple method to separate five bioactive compounds from A. pilosa Ledeb, and it could provide a new idea for the purification of bioactive compounds from other medicinal plants. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)