| Cell Research: |
| Chemistry of Natural Compounds, 2008, 44(3):296-300. | | Isolation, structure elucidation, and mutagenicity of four alternariol derivatives produced by the mangrove endophytic fungus No. 2240[Reference: WebLink] |
METHODS AND RESULTS:
A new Alternariol derivative, 2240B (1), together with Alternariol (2), Alternariol 4,10-dimethyl ether (3), and Alternariol 4-methyl ether (4), was isolated from the ethyl acetate extract of the liquid medium GYT of No. 2240, the mangrove endophytic fungus from the South China Sea Coast. The structure of compound 1 was unambiguously elucidated as Alternariol 4-methyl-10-acethyl ester by spectra including one/two-dimensional NMR, HREIMS, IR, and UV. The structures of compounds 2–4 were also established by spectroscopic analyses and comparison with related literature data.
CONCLUSIONS:
The anticancer tests showed that compounds 2 and 4 had strong activities against KB and KBv200 cells with IC50 values of 3.17, 3.12, and 4.82, 4.94 μg/mL, while compounds 1 and 3 exhibited weak activities against the two kinds of tumor lines with IC50 values of more than 50 μg/mL. | | Toxicol Lett. 2013 May 10;219(1):8-17. | | Alternariol induces abnormal nuclear morphology and cell cycle arrest in murine RAW 264.7 macrophages.[Pubmed: 23454835 ] | The mycotoxin Alternariol (AOH), a frequent contaminant in fruit and cereal products, is known to induce DNA damage with subsequent cell cycle arrest.
METHODS AND RESULTS:
Here we elucidated the effects of AOH on stages of cell cycle progression using the RAW 264.7 macrophage model. AOH resulted in an accumulation of cells in the G2/M-phase (4N). Most cells exhibited a large G2 nucleus whereas numbers of true mitotic cells were reduced relative to control. Both cyclin B1 and p-cdc2 levels increased, while cyclin B1 remained in the cytoplasm; suggesting arrest in the G2/M transition point. Remarkably, after exposure to AOH for 24h, most of the cells exhibited abnormally shaped nuclei, as evidenced by partly divided nuclei, nuclear blebs, polyploidy and micronuclei (MN).
CONCLUSIONS:
AOH treatment also induced abnormal Aurora B bridges, suggesting that cytokinesis was interfered within cells undergoing karyokinesis. A minor part of the resultant G1 tetraploid (4N) cells re-entered the S-phase and progressed to 8N cells. |
|
| Structure Identification: |
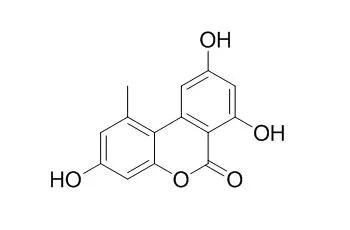
| Experientia. 1985 Sep 15;41(9):1188-90. | | Alternariol, a dibenzopyrone mycotoxin of Alternaria spp., is a new photosensitizing and DNA cross-linking agent.[Pubmed: 3899714] | The mycotoxin Alternariol (3,4',5-trihydroxy-6'-methyldibenzo [a] pyrone) but not Alternariol monomethyl ether (3,4'-dihydroxy-5-methoxy-6'-methyldibenzo [a] pyrone) is phototoxic to Escherichia coli in the presence of near UV light (320-400 nm).
METHODS AND RESULTS:
The phototoxicity bioassays with a DNA repair-deficient mutant of E. coli suggested that DNA may be the molecular target for photo-induced toxicity of Alternariol. Interactions between Alternariol and double-stranded, supercoiled DNA suggest that Alternariol interacts with DNA by intercalation. No DNA breakage was detected in this system; however, Alternariol forms a complex and cross-links double-stranded DNA in near UV light.
CONCLUSIONS:
These results suggest that Alternariol is a new phototoxic, DNA-intercalating agent and is a DNA cross-linking mycotoxin in near UV light. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)