| Animal Research: |
| Journal of Xinxiang Medical College,2012, 29(11): 813-4. | | Effect of arteannuin on expression of tumor necrosis factor-α in rats with ostarthritis.[Reference: WebLink] |
To observe the effect of arteannuin on the level of tumor necrosis factor-α(TNF-α) in rats with ostarthritis.
METHODS AND RESULTS:
Thirty-two rats with ostarthritis were randomly divided into control group,high-dose arteannuin group,media-dose arteannuin group and low-dose arteannuin group,eight rats in each group.The rats in control group were given with saline 20 mL by gavage,once a day.The rats in high-dose arteannuin group,media-dose arteannuin group and low-dose arteannuin group were given with arteannuin 400,300 and 200 mg·kg-1 respectively by gavage,once a day for seven weeks(the arteannuin was dissolved in 20 mL saline).The level of TNF-α was detected by radioimmunoassay(RIA),and the expressions of TNF-α mRNA and protein were detected by reverse transcription-polymerase chain reaction and Western blotting.The levels of serum TNF-α in high-dose,media-dose and low-dose arteannuin groups were decreased significantly than that of control group(P0.05).The level of serum TNF-α in low-dose arteannuin group was higher significantly than those in high-dose and media-dose arteannuin group(P0.05),and the level of serum TNF-α in the media-dose group was higher significantly than that in high-dose arteannuin group(P0.05).There was no statistically significant difference in the expression of TNF-α mRNA between the media-dose and low-dose arteannuin group(P0.05).The expression of TNF-α mRNA in high-dose arteannuin group was lower significantly than those in media-dose and low-dose arteannuin group(P0.05).The expression of TNF-α protein in high-dose and media-dose arteannuin group were lower significantly than those in control group and low-dose arteannuin group(P0.05),but there was no statistically significant difference in the expression of TNF-α protein between media-dose and high-dose arteannuin group(P0.05).
CONCLUSIONS:
Arteannuin can degrade the level of TNF-α in rats with ostarthritis,and effectively inhibit the inflammation. |
|
| Structure Identification: |
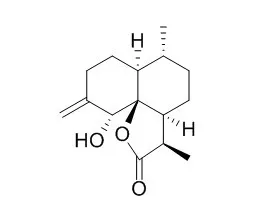
| Tetrahedron.2001 Oct;57(40):8481–8493. | | Structure elucidation of arteannuin O, a novel cadinane diol from Artemisia annua, and the synthesis of arteannuins K, L, M and O.[Reference: WebLink] | The novel cadinane diol, arteannuin O (1), has been obtained from Artemisia annua and its structure has been established by 2D NMR and X-ray crystallography.
METHODS AND RESULTS:
A reconstructive synthesis of arteannuin O from artemisinin is described, which also yields the natural products arteannuin K and Arteannuin L. Mechanistic considerations have led to the conclusion that the stereochemistry of the 5-hydroxyl group was wrongly assigned when arteannuin K, Arteannuin L and M were first reported as natural products.
CONCLUSIONS:
This was confirmed by derivatization of synthetic arteannuin K, Arteannuin L and M as their Mosher esters. | | Acta Chimica Sinica,1989, 47(4):340-4. | | Studies on the Structure and Synthesis of Arteannuin and Related Compound XXII. The Regioselective Synthesis of Arteannuin D[Reference: WebLink] | Arteannuin D (3) coexists with arteannuin (1), which in an antimalarial principle isolated from Chinese medicinal herb Artemisia annua L.
METHODS AND RESULTS:
In this paper the regioselective synthesis of 3 is reported. The aldehyde-ketone 8 obtained from arteannuinic acid (7), was treated with 1 eq trimethylorthoformate in methanol in the presence of a catalytic amount of p-TsOH to provide 9 and 10 in 86% yield in the ratio of 1 to 1, and with 2 eq. of the orthoformate under the same condition to give only 9 in 88% yield. However, pyrolysis of 9 in xylene did not give the desired intermediate 5, but gave 11 and 12 in the yields of 34% and 30%, respectively. Another approach to the synthesis of 3 is to use enol-silyl ether 13 obtained from 8 as a key intermediate through the following sequence of reactions: 8→10→12→13 in 28% overall yield. Trimethylsilyl enol ether 13 was hydroxylated with N-methyl morpholine N-oxide (NMMNO) and a catalytic amount of OsO_4 to give the 3-OH product 14 regioselectively in 52% yield. While the compound 12 was hydroxylated with OsO_4 and NMMNO, followed by cyolization with 10% K_2CO_3 to produce deoxyarteannuin (2) in 72% yield. After protection of 3-OH of 14 by acetylation, it was hydroxylated with a stoichiometric amount of Os0_4 followed by cyclization with 10% K_2CO_3 to give a mixture of 3 and its 3-OH epimer 15 in 59% yield, which after column chromatography gave 3, m. p. 190--192℃, whose spectroscopic data were identical with those reported in the literature. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)