| In vitro: |
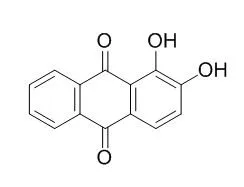
| J Orthop Res. 2012 Sep;30(9):1486-92. | | The natural compound Alizarin as an osteotropic drug for the treatment of bone tumors.[Pubmed: 22411621] | Despite significant clinical improvements, conventional therapies for bone cancer treatment are limited by significant systemic toxicity and lack of specific targeting. In this study, we considered Alizarin, a natural hydroxyanthraquinone derived from madder root with high affinity to calcium and remarkable osteotropic features, as a novel approach for bone cancer treatment. Due to its antitumor properties, as demostrated in colon cancer cells, and to its tropism to bone, Alizarin may be an ideal drug to reduce bone tumor growth.
METHODS AND RESULTS:
We demonstrated that low dosages of Alizarin strongly inhibited the osteosarcoma (IC(50) for Saos-2, MG-63, and U-2 OS cells, 27.5, 29.0, and 69.9 µg/ml, respectively) and breast carcinoma (IC(50) for MDA-MB-231 cells, 62.1 µg/ml) cell proliferation in vitro. Importantly, Alizarin had a significantly lower inhibitory activity on normal cells (IC(50) for MSC, 828.6 µg/ml), thereby revealing a selective activity towards malignant cells. Furthermore, we found that Alizarin acted through the inhibition of ERK phosphorylation and cell cycle arrest in the S-phase. Finally, Alizarin significantly and strongly impaired both osteosarcoma and breast cancer tumorigenesis.
CONCLUSIONS:
Our results highlight a selective and effective inhibitory activity of Alizarin towards cancerous cells, laying the basis for further studies to investigate its application in bone cancer therapy. | | J Phys Chem B . 2017 Apr 27;121(16):4129-4136. | | Ultrafast Intramolecular Proton Transfer of Alizarin Investigated by Femtosecond Stimulated Raman Spectroscopy[Pubmed: 28375609] | | Abstract
We report time-resolved stimulated Raman spectra of Alizarin in DMSO solution with 403 nm excitation. Upon photoexcitation, the intramolecular proton transfer reaction of Alizarin occurs in 70-80 fs, which is confirmed by both the population growth and the frequency and bandwidth changes of skeletal vibrational modes of Alizarin. Interestingly, the vibrational frequencies of ν(C═C) and ν(C═O) show opposite shifts during the reaction, which may implicate changes in the resonance structure of anthraquinone and the attached carbonyl group. Vibrational relaxation in the potential surface of the proton transferred tautomer of Alizarin and the population decay occurring with two distinct time scales were also observed in addition to the solvation dynamics of DMSO solvent molecules. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)