| Kinase Assay: |
| Phytochemistry, 1992, 31(3):757-60. | | Inhibition of Δ5-desaturase in polyunsaturated fatty acid biosynthesis by (−)-asarinin and (−)-epiasarinin.[Reference: WebLink] |
METHODS AND RESULTS:
An extract of Chinese medicine, saishin (Asiasari radix), was found to increase the mycelial dihomo-γ-linolenic acid content of an arachidonic acid-producing fungus, Mortierelia alpina, with an accompanying decrease in its arachidonic acid content.
The factors responsible for this phenomenon were isolated and identified as (−)-asarinin and (−)-epiasarinin, which are the enantiomers of (+)-episesamin and (+)-sesamin, respectively. The inhibitory effects on the Δ5-desaturase in rat liver microsomes of these factors were in the order of (+)-sesamin > (−)-epiasarinin > (−)-asarinin > (+)-episesamin.
CONCLUSIONS:
Kinetic analysis showed that (−)-asarinin and (−)-epiasarinin are non-competitive inhibitors, the Kis for rat liver Δ5-desaturase being 2.8 × 10−4 and 7.1 × 10−4 M, respectively, which are almost the same as the values of the (+)-enantiomers. |
|
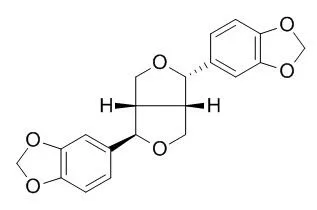
| Structure Identification: |
| Acta Crystallogr C. 2013 Jan;69(Pt 1):87-9. | | (±)-Asarinin.[Pubmed: 23282922] | Asarinin, C(20)H(18)O(6), was isolated as a racemate from the shrub Zanthoxylum alatum.
METHODS AND RESULTS:
Both forms of the enantiomerically pure substance, (+)- and (-)-Asarinin, have been the subject of a total of five previous structure determinations that are essentially identical except for the absolute stereochemistry. However, there seems to be some confusion in the literature concerning these structure determinations of asarinin and also those of its stereoisomer sesamin. The molecular structure of racemic asarinin differs from that of the pure enantiomers in the orientation of one ring system.
CONCLUSIONS:
In the packing of the racemate, molecules are linked by C-H...O interactions to form ribbons parallel to [101]. | | Food Chem., 2011, 124(3):895-9. | | A new mammalian lignan precursor, asarinin.[Reference: WebLink] | Enterolactone (ENL) and enterodiol are mammalian lignans.
METHODS AND RESULTS:
Several plant lignans have been reported as precursors of mammalian lignans. However, asarinin (AS), a furofuran type lignan which occurs in medicinal plants and foods, has not been reported as mammalian lignan precursor to date. After the incubation of AS with human intestinal microflora, AS was converted to not only ENL, but also two more metabolites (mono-demethylenated and ring-cleaved compounds). Under the same conditions, sesamin (SM) was converted to ENL. Furthermore, chiral HPLC analysis showed that ENL produced from AS and SM was (−)-ENL.
CONCLUSIONS:
This is the first report which shows that AS is a mammalian lignan precursor. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)