| In vitro: |
| Anticancer Agents Med Chem. 2013 Jun;13(5):801-10. | | Computational and functional analysis of the androgen receptor antagonist atraric acid and its derivatives.[Pubmed: 23194423] | Androgen receptor (AR) antagonists are important compounds for the treatment of prostate cancer (PCa). The Atraric acid (AA), a natural compound, binds to the AR and acts as a specific AR antagonist. Interestingly, Atraric acid represents a novel chemical platform that could serve as a potential basis for new AR antagonists.
METHODS AND RESULTS:
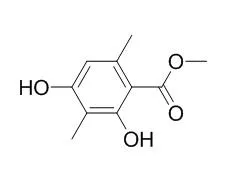
Therefore, one objective of this study was to analyze the chemical/structural requirements for AR antagonism and to obtain predictions of where and how Atraric acid binds to the AR. Further, this study describes the chemical synthesis of 12 Atraric acid derivatives and their analysis using a combination of computational and functional assays. Functional analysis of Atraric acid derivatives indicated that none activated the AR. Both the para-hydroxyl group and the benzene ortho- and the meta-methyl groups of Atraric acid appeared to be essential to antagonize androgen-activated AR activity. Furthermore, extension of the hydrophobic side chain of Atraric acid led to slightly stronger AR antagonism. In silico data suggest that modifications to the basic Atraric acid structure change the hydrogen-bonding network with the AR ligand binding domain (LBD), so that the para-hydroxyl group of Atraric acid forms a hydrogen bond with the LBD, confirming the functional importance of this group for AR antagonism. Moreover, in silico modeling also suggested that the ortho- and meta- methyl groups of Atraric acid interact with hydrophobic residues of the ligand pocket of AR, which might explain their functional importance for antagonism.
CONCLUSIONS:
Thus, these studies identify the chemical groups of Atraric acid that play key roles in allowing the Atraric acid-based chemical platform to act as an AR antagonist. | | Mycology An International Journal on Fungal Biology, 2011, 2(1):18-23. | | PTP1B inhibitory secondary metabolites from the Antarctic lichen Lecidella carpathica.[Reference: WebLink] | Protein tyrosine phosphatase 1B (PTP1B) is an attractive therapeutic target for diabetes, playing a major role in negative regulation of the insulin signaling pathway. Bioassay-guided investigations of an MeOH extract of the Antarctic lichen, Lecidella carpathica, afforded three PTP1B inhibitory metabolites: hopane-6α,22-diol (1), brialmontin 1 (2), and Atraric acid (3), along with two aromatic metabolites (4 and 5) previously isolated from a different Antarctic lichen species.
METHODS AND RESULTS:
Their structures were determined by analysis of NMR and MS data. Compounds 1–3 inhibited PTP1B activity in a dose-dependent manner with IC50 values of 3.7, 14.0 and 51.5 μM, respectively, and kinetic analyses of PTP1B inhibition by compounds 1 and 2 suggested that these compounds inhibit PTP1B activity in a competitive manner. In addition, 6,22-hopanediol (1) displayed some selectivity toward PTP1B over other protein tyrosine phosphatases, such as TCPTP (IC50 = 8.4 μM), SHP-2 (IC50 > 68 μM), LAR (IC50 > 68 μM), and CD45 (IC50 > 68 μM). |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)