| In vitro: |
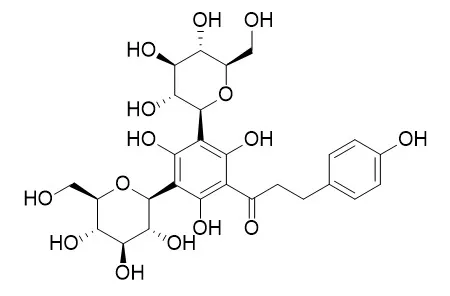
| Food Chem . 2014 Oct 1;160:292-7. | | Biochemical and antimicrobial activity of phloretin and its glycosilated derivatives present in apple and kumquat[Pubmed: 24799241] | | Phloretin and its glycosylated derivatives (phlorizin and Phloretin 3',5'-Di-C-glucoside) are dihydrochalcones that have many interesting biological properties. The results obtained showed that the dihydrochalcones are able to inhibit growth of Gram positive bacteria, in particular Staphylococcus aureus ATCC 6538, Listeria monocytogenes ATCC 13932 and methicillin-resistant S. aureus clinical strains. Moreover, phloretin is active also against the Gram negative bacteria Salmonella typhimurium ATCC 13311. The determination of the enzymatic activity of key metabolic enzymes allowed us to shed some light on the biochemical mechanism of aglycon cell growth inhibition, showing as it remarkably influences the energetic metabolism of S. aureus. In addition, structure/activity determinations highlighted that the presence of a glycosyl moiety bound to the chalcone structure dramatically decreases the antimicrobial activity of phloretin. | | Molecules 2012, 17(12), 14602-14624; | | Food Ingredient Extracts of Cyclopia subternata (Honeybush): Variation in Phenolic Composition and Antioxidant Capacity[Reference: WebLink] | | Cyclopia subternata plants are traditionally used for the production of the South African herbal tea, honeybush, and recently as aqueous extracts for the food industry. A C. subternata aqueous extract and mangiferin (a major constituent) are known to have anti-diabetic properties. Variation in phenolic composition and antioxidant capacity is expected due to cultivation largely from seedlings, having implications for extract standardization and quality control. Aqueous extracts from 64 seedlings of the same age, cultivated under the same environmental conditions, were analyzed for individual compound content, total polyphenol (TP) content and total antioxidant capacity (TAC) in a number of assays. An HPLC method was developed and validated to allow quantification of xanthones (mangiferin, isomangiferin), flavanones (hesperidin, eriocitrin), a flavone (scolymoside), a benzophenone (iriflophenone-3-C-β-glucoside) and dihydrochalcones (phloretin-3',5'-di-C-β-glucoside, 3-hydroxyphloretin-3',5'-di-C-hexoside). Additional compounds were tentatively identified using mass spectrometric detection, with the presence of the 3-hydroxyphloretin-glycoside, an iriflophenone-di-O,C-hexoside, an eriodictyol-di-C-hexoside and vicenin-2 being demonstrated for the first time. Variability of the individual phenolic compound contents was generally higher than that of the TP content and TAC values. Among the phenolic compounds, scolymoside, hesperidin and iriflophenone-3-C-β-glucoside contents were the most variable. A combination of the measured parameters could be useful in product standardization by providing a basis for specifying minimum levels. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)