| In vitro: |
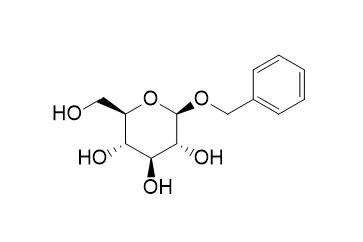
| Nat Prod Res . 2021 Apr;35(8):1388-1392. | | Phenolic glycosides from Nitraria sibirica leaves and their in vitro biological activities[Pubmed: 31379199] | | Seventeen phenolic glycosides were isolated from the Nitraria sibirica. Their structures were identified by the spectroscopic data and comparison with literatures as isovanillyl alcohol-7-O-β-d-glucopyranoside (1), benzyl β-primeveroside (2), benzyl-O-β-d-glucopyranoside (3), 1-O-β-d-glucopyranosyl-4-(8-hydroxyethyl)-2-methoxyphenyl (4), dehydrosyringin (5), trans-ferulic acid-4-O-β-d-glucoside (6), cis-ferulic acid 4-O-β-d-glucopyranoside (7), glucosyringic acid (8), 1-O-feruloyl-β-d-glucoside (9), sachaloside VII (10), (3S, 5R, 6R, 7E, 9S)-megastigmane-7-ene-3-hydroxy-5,6-epoxy-9-O-β-d-glucopyranoside(11), 3-hydroxy-4,5-dimethoxybenzyl alcohol (12), pinoresinol 4-O-β-d-glucopyranoside (13), eucommin A (14), isoeucommin A (15), acanthoside (16), liriodendrin (17). All these compounds except compound 13 were isolated from the Nitraria genus for the first time. In bioactivity assays for all compounds, the compounds 8 and 15 were exhibited strong antioxidant activity (IC50 = 18.11 and 16.29 μM respectively), while compounds 3 and 11 were exhibited strong PTP1B enzymatic inhibition (IC50 = 6.97 and 11.76 μM, respectively). Furthermore, the compounds 10 and 17 were presented strong inhibitory capacities against Candida albicans (14.5 and 13.5 mm, respectively). | | Carbohydr Res . 2008 Nov 24;343(17):2939-2946. | | Engineering of glucoside acceptors for the regioselective synthesis of beta-(1-->3)-disaccharides with glycosynthases[Pubmed: 18828996] | | Glycosynthase mutants obtained from Thermotogamaritima were able to catalyze the regioselective synthesis of aryl beta-D-Galp-(1-->3)-beta-D-Glcp and aryl beta-D-Glcp-(1-->3)-beta-D-Glcp in high yields (up to 90 %) using aryl beta-D-glucosides as acceptors. The need for an aglyconic aryl group was rationalized by molecular modeling calculations, which have emphasized a high stabilizing interaction of this group by stacking with W312 of the enzyme. Unfortunately, the deprotection of the aromatic group of the disaccharides was not possible without partial hydrolysis of the glycosidic bond. The replacement of aryl groups by benzyl ones could offer the opportunity to deprotect the anomeric position under very mild conditions. Assuming that benzyl acceptors could preserve the stabilizing stacking, benzyl beta-d-glucoside firstly assayed as acceptor resulted in both poor yields and poor regioselectivity. Thus, we decided to undertake molecular modeling calculations in order to design which suitable substituted benzyl acceptors could be used. This study resulted in the choice of 2-biphenylmethyl beta-D-glucopyranoside. This choice was validated experimentally, since the corresponding beta-(1-->3) disaccharide was obtained in good yields and with a high regioselectivity. At the same time, we have shown that phenyl 1-thio-beta-D-glucopyranoside was also an excellent substrate leading to similar results as those obtained with the O-phenyl analogue. The NBS deprotection of the S-phenyl group afforded the corresponding disaccharide quantitatively. | | Carbohydr Res . 1989 Dec 1;194:185-198. | | Selective bromoacetylation of alkyl hexopyranosides: a facile preparation of intermediates for the synthesis of (1----6)-linked oligosaccharides[Pubmed: 2620299] | | Bromoacetylation of methyl beta-D-galacto- (1), alpha-D-galacto- (6), beta-D-gluco- (18), (22), and alpha-D-manno-pyranoside (31), and Benzyl beta-D-glucopyranoside (27), gave the corresponding 6-O-bromoacetyl derivatives 2, 7, 19, 23, 32, and 28 in 50-60% yields. Bromoacetylation of methyl 3-O-benzyl-beta-D-galactopyranoside (11) afforded methyl 3-O-benzyl-6-O-bromoacetyl-beta-D-galactopyranoside (12, 60%) as well as methyl 3-O-benzyl-2,6-di-O-bromoacetyl-beta-D-galactopyranoside (13, 14%). Compounds 2, 7, 19, 23, 32, 28, and 12 were benzoylated and the fully protected derivatives obtained were dehaloacetylated with thiourea to afford the methyl 2,3,4-tri-O-benzoyl-D-glycopyranosides of beta-galactose (5), alpha-galactose (9), beta-glucose (21), alpha-glucose (25), and alpha-mannose (34), as well as benzyl 2,3,4-tri-O-benzoyl-beta-D-glucopyranoside (30) and methyl 3-O-benzyl-2,4-di-O-benzoyl-beta-D-galactopyranoside (15). These compounds can be used as nucleophiles for the synthesis of (1----6)-linked oligosaccharides. The conversion 1----5 could be performed without isolation of the intermediates. The treatment of bromoacetyl derivatives with benzoyl chloride in pyridine resulted in the benzoylation of the remaining free hydroxyl groups and the simultaneous substitution of bromine by chlorine, yielding the corresponding mono-O-chloroacetyl derivatives. Benzoylations with benzoyl bromide avoided this secondary event. Glycosyl donors differentially substituted to allow further extension of the oligosaccharide chain at position 6 of D-glucose, D-galactose, and D-mannose, and sequentially at positions 6 and 3 in the case of the D-galactosyl donor derived from 15, were readily obtained by treatment of the appropriate, fully protected methyl glycosides with 1,1-dichloromethyl methyl ether in the presence of a catalytic amount of zinc chloride. | | Bioorg Med Chem Lett . 2014 Sep 1;24(17):4120-4124. | | A new phenylpropanoid and an alkylglycoside from Piper retrofractum leaves with their antioxidant and α-glucosidase inhibitory activity[Pubmed: 25127165] | | Two new compounds, piperoside (1) and isoheptanol 2(S)-O-β-D-xylopyranosyl (1→6)-O-β-D-glucopyranoside (11), along with 10 known compounds 3,4-dihydroxyallylbenzene (2), 1,2-di-O-β-D-glucopyranosyl-4-allylbenzene (3), tachioside (4), benzyl-O-β-D-glucopyranoside (5), icariside F2 (6), dihydrovomifoliol-3'-O-β-D-glucopyranoside (7), isopropyl O-β-D-glucopyranoside (8), isopropyl primeveroside (9), n-butyl O-β-D-glucopyranoside (10), isoheptanol 2(S)-O-β-D-apiofuranosyl-(1→6)-O-β-D-glucopyranoside (12), were isolated from the leaves of Piper retrofractum. Their structures were determined from 1D-NMR, 2D-NMR, and HR-ESI-MS spectral, a modified Mosher's method, and comparisons with previous reports. All of the isolated compounds showed modest α-glucosidase inhibitory (4.60±1.74% to 11.97±3.30%) and antioxidant activities under the tested conditions. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)