| In vitro: |
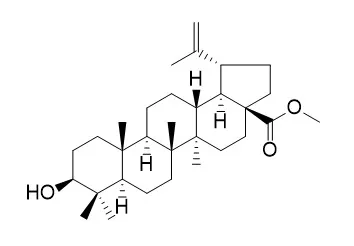
| Parasitol Res. 2009 Jul;105(1):275-9. | | Antimalarial activity of betulinic acid and derivatives in vitro against Plasmodium falciparum and in vivo in P. berghei-infected mice.[Pubmed: 19367418] | Malaria is one of the most important tropical diseases and mainly affects populations living in developing countries. Reduced sensitivity of Plasmodium sp. to formerly recommended antimalarial drugs places an increasing burden on malaria control programs as well as on national health systems in endemic countries. The present study aims to evaluate the antimalarial activity of betulinic acid and its derivative compounds, betulonic acid, betulinic acid acetate, Betulinic acid methyl ester, and Betulinic acid methyl ester acetate.
METHODS AND RESULTS:
These substances showed antiplasmodial activity against chloroquine-resistant Plasmodium falciparum parasites in vitro, with IC(50) values of 9.89, 10.01, 5.99, 51.58, and 45.79 microM, respectively. Mice infected with Plasmodium berghei and treated with betulinic acid acetate had a dose-dependent reduction of parasitemia.
CONCLUSIONS:
Our results indicate that betulinic acid and its derivative compounds are candidates for the development of new antimalarial drugs. | | J Nat Prod. 2002 May;65(5):645-8. | | Differentiation- and apoptosis-inducing activities by pentacyclic triterpenes on a mouse melanoma cell line.[Pubmed: 12027734 ] | In a study to investigate the relationship between the chemical structure and the differentiation-inducing activity of pentacyclic triterpenes, several lupane, oleanane, and ursane triterpenes were prepared and their effects on B16 2F2 melanoma cell differentiation and growth were examined.
METHODS AND RESULTS:
Eleven lupane triterpenes used in this study acted on the melanoma cells as a melanogen, but no induction of melanogenesis of B16 2F2 cells by oleanane and ursane was detected. The differences at C-17 of the lupane series and acetylation of the OH group at C-3 did not markedly influence their activities. However, the ED(50) value for up-regulation of melanin biosynthesis was markedly decreased by the oxidation of the OH group at C-3 of lupeol (1). Betulinic acid (11), its methyl ester (Betulinic acid methyl ester, 12), lup-28-al-20(29)-ene-3beta-ol (9), and lup-28-al-20(29)-en-3-one (10) inhibited B16 2F2 cell proliferation by induction of apoptosis.
CONCLUSIONS:
These findings suggested that the carbonyl group at C-17 might be essential for the apoptotic effects of these compounds on B16 2F2 cells. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)