| In vitro: |
| J Biol Chem. 2014 Apr 4;289(14):9926-35. | | (-)-Epicatechin gallate (ECG) stimulates osteoblast differentiation via Runt-related transcription factor 2 (RUNX2) and transcriptional coactivator with PDZ-binding motif (TAZ)-mediated transcriptional activation.[Pubmed: 24515112] | Osteoporosis is a degenerative bone disease characterized by low bone mass and is caused by an imbalance between osteoblastic bone formation and osteoclastic bone resorption. It is known that the bioactive compounds present in green tea increase osteogenic activity and decrease the risk of fracture by improving bone mineral density. However, the detailed mechanism underlying these beneficial effects has yet to be elucidated.
METHODS AND RESULTS:
In this study, we investigated the osteogenic effect of (-)-Epicatechin gallate (ECG), a major bioactive compound found in green tea. We found that ECG effectively stimulates osteoblast differentiation, indicated by the increased expression of osteoblastic marker genes. Up-regulation of osteoblast marker genes is mediated by increased expression and interaction of the transcriptional coactivator with PDZ-binding motif (TAZ) and Runt-related transcription factor 2 (RUNX2). ECG facilitates nuclear localization of TAZ through PP1A. PP1A is essential for osteoblast differentiation because inhibition of PP1A activity was shown to suppress ECG-mediated osteogenic differentiation.
CONCLUSIONS:
Taken together, the results showed that ECG stimulates osteoblast differentiation through the activation of TAZ and RUNX2, revealing a novel mechanism for green tea-stimulated osteoblast differentiation. |
|
| In vivo: |
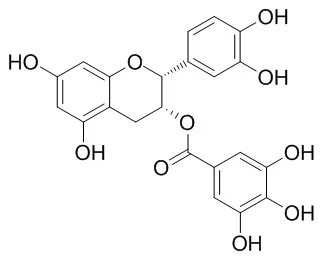
| Biol Pharm Bull . 2003 May;26(5):608-12. | | Pharmacokinetics of (-)-epicatechin-3-O-gallate, an active component of Onpi-to, in rats[Pubmed: 12736498] | | Abstract
(-)-Epicatechin-3-O-gallate (ECG), a component of Rhei Rhizoma, is one of the active components of Onpi-to, a herbal medicine composed of five crude drugs (Rhei Rhizome, Glycyrrhizae Radix, Ginseng Radix, Zingiberis Rhizoma and Aconiti Tuber), which has been used in patients with chronic renal failure. Pharmacokinetics of ECG was investigated in male rats employing an HPLC-electrochemical detection method. 1. Following oral administration of ECG, ECG plasma levels revealed curves characterized by peaks at 0.065, 0.063 and 0.085 h corresponding to dosages of 12.5, 25.0 and 50.0 mg/kg at mean concentrations of 49.62, 212.89 and 464.04 ng/ml, respectively. Plasma levels subsequently declined bi-exponentially. ECG demonstrated nonlinear pharmacokinetics in terms of C(max) and AUC(0-inf). 2. The absolute bioavailability values (F) were 1.02, 1.47 and 3.30% at doses of 12.5, 25.0, and 50.0 mg/kg, respectively. 3. Following intravenous injection of ECG, plasma levels of ECG decreased with the gamma-elimination half-life (t(1/2gamma)) of 4.03 h. 4. Following oral administration of ECG, urinary levels of ECG were lower than the quantitation limit. Moreover, cumulative excretion of the metabolites, delta-(3,4-dihydroxyphenyl)-gamma-valerolactone and delta-(3-methoxy-4-hydroxyphenyl)-gamma-valerolactone, was 2.45 and 0.23% of dose, respectively, up to 30 h after dosing. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)