| Description: |
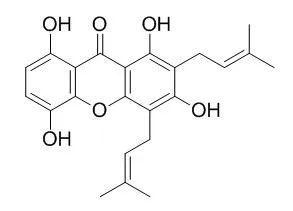
Gartanin is an androgen receptor degradation enhancer, it is also a potential neuroprotective agent against glutamate-induced oxidative injury partially through increasing Nrf-2-independed HO-1 and AMPK/SIRT1/PGC-1αsignaling pathways. Gartanin possesses potent antioxidant, anti-inflammatory, antifungal and antineoplastic properties, it has anti-proliferation effect in T98G cells, which is most likely via cell cycle arrest modulated by autophagy, which is regulated by PI3K/Akt/mTOR signalling pathway, while its anti-migration effect is most likely via suppression of MMP-2/-9 activity which is involved in MAPK signalling pathway. |
| In vitro: |
| Nutr Cancer. 2013;65 Suppl 1:68-77. | | The effect of gartanin, a naturally occurring xanthone in mangosteen juice, on the mTOR pathway, autophagy, apoptosis, and the growth of human urinary bladder cancer cell lines.[Pubmed: 23682785] | Androgen receptor (AR) has been a target of prostate cancer for nearly seven decades. In the last several years there has been an interest in identifying compounds that promote degradation of the androgen receptor. In the present study, Gartanin, an isoprentylated xanthone in the mangosteen fruit, was evaluated for enhancing AR degradation, and inducing the unfolded protein response pathway.
METHODS AND RESULTS:
The interaction of Gartanin with the ligand-binding domain was characterized using a fluorescence polarization cell-free assay and cell-based FRET assay. Western blot analysis identified modulation of ER stress markers (BiP, PERK, IRE1, and CHOP) along with androgen receptor degradation. A computation simulation was performed to identify possible orientations of Gartanin with the ligand-binding domain. Utilizing a cell-free and cell-based FRET assays Gartanin was found to interact with the ligand-binding domain through a solely antagonist interaction. Interestingly, inhibition of CHOP, a critical component of the ER stress pathway, was observed to stabilize AR.
CONCLUSIONS:
Gartanin is an isoprenylated xanthone that promotes AR degradation with evidence suggesting this process is critically regulated by the unfolded protein response pathway. | | J Cell Mol Med. 2017 Jan;21(1):46-57. | | Gartanin induces cell cycle arrest and autophagy and suppresses migration involving PI3K/Akt/mTOR and MAPK signalling pathway in human glioma cells.[Pubmed: 27491646 ] | In central nervous system, glioma is the most common primary brain tumour. The diffuse migration and rapid proliferation are main obstacles for successful treatment.
METHODS AND RESULTS:
Gartanin, a natural xanthone of mangosteen, suppressed proliferation, migration and colony formation in a time- and concentration-dependent manner in T98G glioma cells but not in mouse normal neuronal HT22 cells.
Gartanin, at low micromole, led to cell cycle arrest in G1 phase accompanied by inhibited expression level of G1 cell cycle regulatory proteins cyclin D1, while increased expression level of cyclin-dependent kinase inhibitor p27Kip1. In addition, the secretion and activity of matrix metalloproteinases 2/9 (MMP-2/-9) were significantly suppressed in T98G cells treated with Gartanin, and it might result from modulating mitogen-activated protein kinases (MAPK) signalling pathway in T98G glioma cells. Moreover, Gartanin significantly induced autophagy in T98G cells and increased GFP-LC3 punctate fluorescence accompanied by the increased expression level of Beclin 1 and LC3-II, while suppressed expression level of p62. Gartanin treatment resulted in obvious inhibition of PI3K/Akt/mTOR signalling pathway, which is important in modulating autophagy. Notably, Gartanin-mediated anti-viability was significantly abrogated by autophagy inhibitors including 3-methyladenine (3-MA) and chloroquine (CQ).
CONCLUSIONS:
These results indicate that anti-proliferation effect of Gartanin in T98G cells is most likely via cell cycle arrest modulated by autophagy, which is regulated by PI3K/Akt/mTOR signalling pathway, while anti-migration effect is most likely via suppression of MMP-2/-9 activity which is involved in MAPK signalling pathway. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)