| In vitro: |
| Bioorg Med Chem. 2013 Dec 15;21(24):7591-4. | | Bioactive compounds from Stuhlmannia moavi from the Madagascar dry forest.[Pubmed: 24239390 ] |
METHODS AND RESULTS:
Bioassay-directed fractionation of the leaf and root extracts of the antiproliferative Madagascar plant Stuhlmannia moavi afforded 6-acetyl-5,8-dihydroxy-2-methoxy-7-methyl-1,4-naphthoquinone (stuhlmoavin, 1) as the most active compound, with an IC50 value of 8.1 μM against the A2780 human ovarian cancer cell line, as well as the known homoisoflavonoid Bonducellin (2) and the stilbenoids 3,4,5'-trihydroxy-3'-methoxy-trans-stilbene (3), piceatannol (4), resveratrol (5), rhapontigenin (6), and isorhapontigenin (7). The structure elucidation of all compounds was based on NMR and mass spectroscopic data, and the structure of 1 was confirmed by a single crystal X-ray analysis.
CONCLUSIONS:
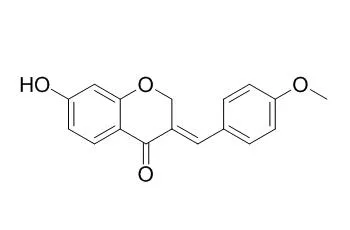
Compounds 2-5 showed weak A2780 activities, with IC50 values of 10.6, 54.0, 41.0, and 74.0 μM, respectively. Compounds 1-3 also showed weak antimalarial activity against Plasmodium falciparum with IC50 values of 23, 26, and 27 μM, respectively. | | Eur J Med Chem. 2013 Aug;66:499-507. | | 7-Hydroxy-(E)-3-phenylmethylene-chroman-4-one analogues as efflux pump inhibitors against Mycobacterium smegmatis mc2 155.[Pubmed: 23832254] | Efflux pump (EP) induces resistance in mycobacteria and hence could be explored as a new target for the discovery of anti-TB agents. In search for efflux pump inhibitors from natural products, Bonducellin, a homoisoflavonoid was isolated from Caesalpinia digyna roots and evaluated for modulation and EP inhibitory activity.
METHODS AND RESULTS:
Bonducellin showed modulation in the MIC of EtBr by eight fold at a concentration of 62.5 mg/L and also showed significant EP inhibitory activity. A synthetic scheme was designed to prepare analogues of 7-hydroxy-(E)-3-phenylmethylene-chroman-4-one by modification at the phenylmethylene-ring and the synthesized compounds were evaluated in accumulation and efflux assays.
CONCLUSIONS:
Analogues 1, 7-11, 13-15, 17 and 19 were found to be good modulators and decreased the MIC of EtBr by ≥4 fold at sub-inhibitory concentration. The compounds 8, 13 and 17 were the most potent inhibitors of ethidium bromide efflux in Mycobacterium smegmatis mc(2) 155. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)