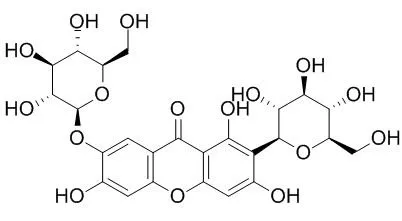
| Description: |
Neomangiferin exhibits antidiabetic and antiosteoporotic actions; it has beneficial effects on high fat diet-induced nonalcoholic fatty liver disease in rats, it also modulates the Th17/Treg balance and ameliorates colitis in mice. |
| Targets: |
PPAR | LDL | TNF-α | NF-kB | IL Receptor | COX | NOS | Fatty Acid Synthase |
| In vitro: |
| Phytomedicine. 2016 Feb 15;23(2):131-40. | | Neomangiferin modulates the Th17/Treg balance and ameliorates colitis in mice.[Pubmed: 26926174 ] | Anemarrhena asphodeloides (Liliaceae family) and Mangifera indica L. (Anacardiaceae family) contain Neomangiferin as the main active constituent and have been used to treat inflammation, asthma, and pain.
A preliminary study found that Neomangiferin inhibited splenic T cell differentiation into Th17 cells and promoted Treg cell production in vitro. Therefore, we examined its anti-colitic effects in vitro and in vivo.
METHODS AND RESULTS:
Splenocytes isolated from C57BL/6J mice were treated with Neomangiferin. Colitis was either induced in vivo by intrarectal administration of 2,4,6-trinitrobenzene sulfonic acid (TNBS) to C57BL/6J mice or occurred spontaneously in colitis caused by interleukin (IL)-10 knockout at age of 13 weeks. Mice were treated daily with Neomangiferin or sulfasalazine. Inflammatory markers, cytokines, enzymes and transcription factors were measured by enzyme-linked immunosorbent assay, immunoblot, and flow cytometry.
Neomangiferin suppressed retinoic acid receptor-related orphan receptor gamma t (RORγt) and IL-17 expression in IL-6/transforming growth factor β-stimulated Th17 splenocytes and increased IL-10 expression in vitro. Mouse TNBS-induced colon shortening, macroscopic score, and myeloperoxidase activity were inhibited by Neomangiferin, which also reduced TNBS-induced activation of nuclear factor-κB and extracellular signal-regulated kinases, as well as expression of inducible nitric oxide synthase and cyclooxygenase-2. In addition, Neomangiferin inhibited TNBS-induced expression of tumor necrosis factor-α, IL-17, IL-6, and IL-1β, and increased IL-10 expression. Neomangiferin inhibited TNBS-induced differentiation to Th17 cells and promoted the development of Treg cells. Moreover, in IL-10(-/-) mice, Neomangiferin inhibited colonic myeloperoxidase activity, suppressed Th17 cell differentiation, and reduced levels of TNF-α and IL-17.
CONCLUSIONS:
Neomangiferin may restore the balance between Th17/Treg cells by suppressing IL-17 and RORγt expression and inducing IL-10 and forkhead box P3 expression, thus ameliorating colitis. |
|
| In vivo: |
| Int Immunopharmacol. 2015 Mar;25(1):218-28. | | Beneficial effects of neomangiferin on high fat diet-induced nonalcoholic fatty liver disease in rats.[Pubmed: 25661699] | This study was carried out to determine the effect and mechanism of action of Neomangiferin (NG) on high-fat diet-induced nonalcoholic fatty liver disease (NAFLD) in rats.
METHODS AND RESULTS:
NAFLD rats were randomly assigned into several groups of equal number. NG (50, 25mg/kg·day(-1) BW) and lipanthyl (PT, 5mg/kg·day(-1) BW) were given to the NAFLD rats, respectively. In the study, serum lipids, metabolic rate, liver fat, liver lipids and histology were examined. To further investigate the molecular mechanism of the effect of NG on NAFLD, expression levels of mRNA and protein for peroxisome proliferator-activated receptor α (PPARα), fatty acid transport protein 2 (FATP2), long-chain-fatty-acid - CoA ligase 1 (ACSL1) and carnitine palmitoyltransferase 1a (CPT1a) in the liver were determined by Real Time-PCR and western blot analysis, respectively. NG administration significantly reduced the final body weight, liver fat accumulation, and serum triglyceride (TG), total cholesterol (TC) concentrations, low-density lipoprotein cholesterol (LDL-C), glucose (GLU) levels, and hepatic TG, TC, malondialdehyde (MDA) levels, but increased serum high-density lipoprotein cholesterol (HDL-C) and hepatic superoxide dismutase (SOD) levels. NG upregulated the mRNA and protein expression of PPARα and CPT1a, but downregulated the mRNA and protein expression of FATP2 and ACSL1 in the liver.
CONCLUSIONS:
These results suggested that NG can regulate NAFLD partly by modulating the expression levels of genes involved in FFA uptake and lipid oxidation. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)