| Kinase Assay: |
| J Biochem. 2011 Apr;149(4):415-22. | | Calystegine B3 as a specific inhibitor for cytoplasmic alpha-mannosidase, Man2C1.[Pubmed: 21217149] | Development of a specific inhibitor for Man2C1 is critical to understanding its biological significance.
METHODS AND RESULTS:
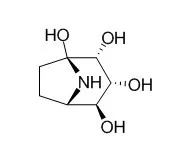
In this study, we identified a plant-derived alkaloid, Calystegine B3, as a potent specific inhibitor for Man2C1 activity. Biochemical enzyme assay revealed that Calystegine B3 was a highly specific inhibitor for Man2C1 among various α-mannosidases prepared from rat liver. Consistent with this in vitro result, an in vivo experiment also showed that treatment of mammalian-derived cultured cells with this compound resulted in drastic change in both structure and quantity of free oligosaccharides in the cytosol, whereas no apparent change was seen in cell-surface oligosaccharides.
CONCLUSIONS:
Calystegine B3 could thus serve as a potent tool for the development of a highly specific in vivo inhibitor for Man2C1. |
|
| Structure Identification: |
| Bioorg Med Chem. 2014 Apr 15;22(8):2435-41. | | Docking and SAR studies of calystegines: binding orientation and influence on pharmacological chaperone effects for Gaucher's disease.[Pubmed: 24657053] | We report on the identification of the required configuration and binding orientation of nor-tropane alkaloid calystegines against β-glucocerebrosidase.
METHODS AND RESULTS:
Calystegine B2 is a potent competitive inhibitor of human lysosomal β-glucocerebrosidase with Ki value of 3.3 μM. A molecular docking study revealed that calystegine B2 had a favorable van der Waals interactions (Phe128, Trp179, and Phe246) and the hydrogen bonding (Glu235, Glu340, Asp127, Trp179, Asn234, Trp381 and Asn396) was similar to that of isofagomine. All calystegine isomers bound into the same active site as calystegine B2 and the essential hydrogen bonds formed to Asp127, Glu235 and Glu340 were maintained. However, their binding orientations were obviously different. Calystegine A3 bound to β-glucocerebrosidase with the same orientations as calystegine B2 (Type 1), while Calystegine B3 and B4 had different binding orientations (Type 2). It is noteworthy that Type 1 orientated calystegines B2 and A3 effectively stabilized β-glucocerebrosidase, and consequently increased intracellular β-glucocerebrosidase activities in N370S fibroblasts, while Type 2 orientated calystegines B3 and B4 could not keep the enzyme activity.
CONCLUSIONS:
These results clearly indicate that the binding orientations of calystegines are changed by the configuration of the hydroxyl groups on the nor-tropane ring and the suitable binding orientation is a requirement for achieving a strong affinity to β-glucocerebrosidase. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)