| Structure Identification: |
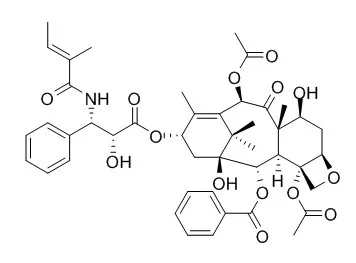
| Fitoterapia. 2013 Oct;90:79-84. | | Synthesis, isolation, stereostructure and cytotoxicity of paclitaxel analogs from cephalomannine.[Pubmed: 23876369] | Four paclitaxel derivatives were afforded by preparative HPLC separation of two pairs of diastereoisomers, which were obtained by catalytic hydrogenation and epoxidation of the C-13 side-chain double bond of Cephalomannine, a naturally occurring paclitaxel analog.
METHODS AND RESULTS:
The four paclitaxel derivatives were analyzed using NMR, CD spectroscopy, and side-chain hydrolysis in order to measure their optical rotations and GC characteristics. In this way, the stereoconfigurations of the products were determined. Evaluation of the compounds' activity indicated that they had differing cytotoxic activities: compound 5 had superior activity in BCG-823 tumor cells compared to paclitaxel, while compound 7 had superior activity in HCT-8 and A549 tumor cells compared to paclitaxel.
CONCLUSIONS:
These results indicate that the stereoconfiguration of the paclitaxel N-acyl side chain has a significant impact on its activity. | | Drug Metab Dispos. 2008 Feb;36(2):418-26. | | Taxane's substituents at C3' affect its regioselective metabolism: different in vitro metabolism of cephalomannine and paclitaxel.[Pubmed: 18039807] | To investigate how taxane's substituents at C3' affect its metabolism, we compared the metabolism of Cephalomannine and paclitaxel, a pair of analogs that differ slightly at the C3' position.
METHODS AND RESULTS:
After Cephalomannine was incubated with human liver microsomes in an NADPH-generating system, two monohydroxylated metabolites (M1 and M2) were detected by liquid chromatography/tandem mass spectrometry. C4'' (M1) and C6alpha (M2) were proposed as the possible hydroxylation sites, and the structure of M1 was confirmed by (1)H NMR. Chemical inhibition studies and assays with recombinant human cytochromes P450 (P450s) indicated that 4''-hydroxyCephalomannine was generated predominantly by CYP3A4 and 6alpha-hydroxyCephalomannine by CYP2C8. The overall biotransformation rate between paclitaxel and Cephalomannine differed slightly (184 vs. 145 pmol/min/mg), but the average ratio of metabolites hydroxylated at the C13 side chain to C6alpha for paclitaxel and Cephalomannine varied significantly (15:85 vs. 64:36) in five human liver samples. Compared with paclitaxel, the major hydroxylation site transferred from C6alpha to C4'', and the main metabolizing P450 changed from CYP2C8 to CYP3A4 for Cephalomannine. In the incubation system with rat or minipig liver microsomes, only 4''-hydroxyCephalomannine was detected, and its formation was inhibited by CYP3A inhibitors. Molecular docking by AutoDock suggested that Cephalomannine adopted an orientation in favor of 4''-hydroxylation, whereas paclitaxel adopted an orientation favoring 3'-p-hydroxylation. Kinetic studies showed that CYP3A4 catalyzed Cephalomannine more efficiently than paclitaxel due to an increased V(m).
CONCLUSIONS:
Our results demonstrate that relatively minor modification of taxane at C3' has major consequence on the metabolism. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)