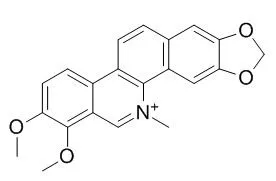
| Description: |
Chelerythrine is a well-known protein kinase C inhibitor, can inhibit telomerase activity, it also can block the human P2X 7 receptor. Chelerythrine has antimanic, potential antiproliferative and antitumor effects, it has significant cytotoxic effect, independent of p53 and androgen status, on human prostate cancer cell lines. |
| Targets: |
PKC | cAMP | Bcl-2/Bax | p53 | Calcium Channel | ATPase | Sodium Channel | DNA/RNA Synthesis | HSP (e.g. HSP90) | p21 |
| In vitro: |
| Tumour Biol. 2014 Jan;35(1):129-40. | | Chelerythrine induces reactive oxygen species-dependent mitochondrial apoptotic pathway in a murine T cell lymphoma.[Pubmed: 23900672] | Chelerythrine is a well-known protein kinase C inhibitor and potential antiproliferative and antitumor pharmacological agent. Chelerythrine inhibits/suppresses the HSF1 phosphorylation by inhibiting PKC and blocks the nuclear migration and subsequent synthesis of hsp70 leading to reduced cell viability and activation of apoptotic machinery. Chelerythrine is also known to enhance the production of reactive oxygen intermediate that is strong activator of apoptosis in high concentration.
METHODS AND RESULTS:
Therefore, the present study intended to investigate the role of Chelerythrine-induced reactive oxygen intermediate on the viability and apoptosis of Dalton's lymphoma cells. Enhanced production of reactive oxygen species in Dalton's lymphoma (DL) cells was observed upon treatment of Chelerythrine only which was seen completely abolished on treatment of mitochondrial complex inhibitors rotenone and malonate, and anti-oxidant, N-acetyl-L-cysteine. Increased number of DL cells undergoing apoptosis, as observed by fluorescent microscopy and flow cytometry analysis, in Chelerythrine only-treated group was seen that was significantly inhibited on treatment of mitochondrial complex inhibitors and anti-oxidants. Staurosporine, on the other hand, does not lead to enhanced production of reactive oxygen intermediate in DL cells. | | Redox Biol . 2017 Aug;12:367-376. | | Induction of reactive oxygen species-stimulated distinctive autophagy by chelerythrine in non-small cell lung cancer cells[Pubmed: 28288416] | | Abstract
Chelerythrine (CHE), a natural benzo[c]phenanthridine alkaloid, shows anti-cancer effect through a number of mechanisms. Herein, the effect and mechanism of the CHE-induced autophagy, a type II programmed cell death, in non-small cell lung cancer (NSCLC) cells were studied for the first time. CHE induced cell viability decrease, colony formation inhibition, and apoptosis in a concentration-dependent manner in NSCLC A549 and NCI-H1299 cells. In addition, CHE triggered the expression of phosphatidylethanolamine-modified microtubule-associated protein light-chain 3 (LC3-II). The CHE-induced expression of LC3-II was further increased in the combination treatment with chloroquine (CQ), an autophagy inhibitor, and large amounts of red-puncta were observed in the CHE-treated A549 cells with stable expression of mRFP-EGFP-LC3, indicating that CHE induces autophagy flux. Silence of beclin 1 reversed the CHE-induced expression of LC3-II. Inhibition of autophagy remarkably reversed the CHE-induced cell viability decrease and apoptosis in NCI-H1299 cells but not in A549 cells. Furthermore, CHE triggered reactive oxygen species (ROS) generation in both cell lines. A decreased level of ROS through pretreatment with N-acetyl-L-cysteine reversed the CHE-induced cell viability decrease, apoptosis, and autophagy. Taken together, CHE induced distinctive autophagy in A549 (accompanied autophagy) and NCI-H1299 (pro-death autophagy) cells and a decreased level of ROS reversed the effect of CHE in NSCLC cells in terms of cell viability, apoptosis, and autophagy.
Keywords: Apoptosis; Autophagy; Chelerythrine; NSCLC; ROS. |
|
| In vivo: |
| Pharmacol Rep. 2014 Aug;66(4):722-5. | | Partial effects of the protein kinase C inhibitor chelerythrine in a battery of tests for manic-like behavior in black Swiss mice.[Pubmed: 24948079] | | The aim of the present study was to test the effects of peripheral (intraperitoneal) administration of Chelerythrine in a battery of mania-related behavioral tests in black Swiss mice, a strain specific battery that was previously demonstrated to distinguish differential effects of mood stabilizing drugs.
RESULTS:
Sub-chronic administration of 1.0mg/kg or 2.0mg/kg Chelerythrine had marginal effects to reduce spontaneous activity and sweet solution preference in black Swiss mice which naturally show mania-like behaviors. Chelerythrine had no effects on the behavior of these mice in the elevated plus-maze, the forced swim test and the amphetamine-induced hyperactivity test.
CONCLUSIONS:
The partial effects in the battery are not unique as previous studies showed that lithium, valproate and risperidone, all used in the treatment of bipolar disorder, have distinct profiles in the battery. It is therefore concluded that Chelerythrine may have antimanic effects and additional dose and time response studies are warranted to further evaluate its range of activity. | | Life Sci . 2019 Jan 1;216:85-91. | | Protein kinase C inhibitor chelerythrine attenuates partial unilateral ureteral obstruction induced kidney injury in neonatal rats[Pubmed: 30439378] | | Abstract
The present study aimed to evaluate the renoprotective effects of Chelerythrine (CHE), a protein kinase C inhibitor, on neonatal rats after partial unilateral ureteral obstruction (UUO) surgery. New born Sprague Dawley rats were subjected to partial UUO 48 h after birth and received a daily intraperitoneal injection of 5 mg/kg CHE. At 21-day age, the rats were scarified and the kidneys were collected for analysis. Results showed that CHE treatment significantly increased kidney weight and restored renal function in the obstructed kidney. Histological examination demonstrated that CHE attenuated renal injury by reducing renal parenchymal loss and preventing glomerular and tubular degeneration. In addition, CHE inhibited partial UUO-induced upregulated kidney injury molecule-1 expression and apoptosis and renal fibrosis. Moreover, as a PKC inhibitor, CHE significantly inhibited PKCα and PKCβ membrane translocation. This action may be associated with its effects of anti-apoptosis and anti-fibrosis and contribute to the renoprotection. This short-term study suggests that CHE is beneficial for obstructive nephropathy in neonatal rats and provides foundation for further studies to reveal the long-term effects of CHE on obstructive nephropathy in children and infants.
Keywords: Chelerythrine; Kidney injury; Neonatal mouse; Protein kinase C inhibitor; Unilateral ureteral obstruction. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)