| In vitro: |
| J Gastroenterol Hepatol. 2013 May;28(5):823-8. | | Acidic deoxycholic acid and chenodeoxycholic acid induce interleukin-8 production through p38 mitogen-activated protein kinase and protein kinase A in a squamous epithelial model.[Pubmed: 23425072] | Immune-mediated mucosal inflammation characterized by the production of interleukin (IL)-8 is associated with the development of gastroesophageal reflux disease. The effects of bile acids, which are major components of reflux fluid, on the production of IL-8 and related mechanisms remain unclear. This study aimed to address these questions using an esophageal stratified squamous epithelial model.
METHODS AND RESULTS:
Normal human esophageal epithelial cells were seeded on the Transwell inserts and cultured with the air-liquid interface system to establish the model. Bile acids under different pH conditions were added to the apical compartment to examine their effects on IL-8 production and the underlying cellular signaling.
Conjugated bile acids under a neutral or acidic condition did not induce IL-8 production, and unconjugated bile acids, deoxycholic acid (DCA), and Chenodeoxycholic acid (CDCA) all significantly induced IL-8 production, dose- and time-dependently, only under weakly acid conditions. Inhibition of p38 mitogen-activated protein kinase (p38 MAPK) and protein kinase A (PKA) attenuated the production of IL-8 induced by acidic DCA and CDCA. Inhibition of PKA did not block the bile acid-induced p38 MAPK activation.
CONCLUSIONS:
Compared with conjugated bile acids, the unconjugated bile acids DCA and CDCA are more likely to induce IL-8 production in vivo, especially under weakly acid conditions. This process involves two independent signaling pathways, p38 MAPK and PKA. |
|
| In vivo: |
| J Inherit Metab Dis. 2014 Sep;37(5):851-61. | | Liver disease in infancy caused by oxysterol 7 α-hydroxylase deficiency: successful treatment with chenodeoxycholic acid.[Pubmed: 24658845] | A child of consanguineous parents of Pakistani origin developed jaundice at 5 weeks and then, at 3 months, irritability, a prolonged prothrombin time, a low albumin, and episodes of hypoglycaemia. Investigation showed an elevated alanine aminotransferase with a normal γ-glutamyl-transpeptidase.
METHODS AND RESULTS:
Analysis of urine by electrospray ionisation tandem mass spectrometry (ESI-MS/MS) showed that the major peaks were m/z 480 (taurine-conjugated 3β-hydroxy-5-cholenoic acid) and m/z 453 (sulphated 3β-hydroxy-5-cholenoic acid). Analysis of plasma by gas chromatography-mass spectrometry (GC-MS) showed increased concentrations of 3β-hydroxy-5-cholenoic acid, 3β-hydroxy-5-cholestenoic acid and 27-hydroxycholesterol, indicating oxysterol 7 α-hydroxylase deficiency. The patient was homozygous for a mutation (c.1249C>T) in CYP7B1 that alters a highly conserved residue in oxysterol 7 α-hydroxylase (p.R417C) - previously reported in a family with hereditary spastic paraplegia type 5. On treatment with ursodeoxycholic acid (UDCA), his condition was worsening, but on Chenodeoxycholic acid (CDCA), 15 mg/kg/d, he improved rapidly. A biopsy (after 2 weeks on CDCA), showed a giant cell hepatitis, an evolving micronodular cirrhosis, and steatosis. The improvement in liver function on CDCA was associated with a drop in the plasma concentrations and urinary excretions of the 3β-hydroxy-Δ5 bile acids which are considered hepatotoxic. At age 5 years (on CDCA, 6 mg/kg/d), he was thriving with normal liver function. Neurological development was normal apart from a tendency to trip. Examination revealed pes cavus but no upper motor neuron signs.
CONCLUSIONS:
The findings in this case suggest that CDCA can reduce the activity of cholesterol 27-hydroxylase - the first step in the acidic pathway for bile acid synthesis. | | J Clin Endocrinol Metab. 2013 Aug;98(8):3351-8. | | Effects of chenodeoxycholic acid on the secretion of gut peptides and fibroblast growth factors in healthy humans.[Pubmed: 23783097] | We hypothesized that intraduodenal infusions of Chenodeoxycholic acid (CDCA) would stimulate FGF and gut peptide secretion, thereby positively influencing glucose homeostasis.
METHODS AND RESULTS:
This randomized, double-blind, placebo-controlled, crossover trial included 12 healthy volunteers who received intraduodenal infusions (2.0 mL/min for 180 minutes) of saline, Chenodeoxycholic acid (5 or 15 mmol/L), and a fatty acid (sodium oleate), either alone or with 5 mmol/L Chenodeoxycholic acid. After 60 minutes, an oral glucose tolerance test (oGTT) was performed. Within the first 60 minutes, high-concentration Chenodeoxycholic acid induced a small but significant increase in GLP-1 and CCK secretion (P = .016 and P =.011), whereas plasma C-peptide, insulin, and glucose were not affected. Attenuated C-peptide and insulin release was observed after the oGTT with 15 mmol/L CDCA (P = .013 and P =.011). Plasma BA and FGF19 levels significantly increased after Chenodeoxycholic acid administration (P = .001 and P < .001).
CONCLUSIONS:
Chenodeoxycholic acid modulates GLP-1 and CCK secretion; the effect is small and does not influence glucose levels. The marked increase in plasma BAs and the attenuated insulin release after the oGTT indicate the role of BAs in glycemic control, independent of the incretin axis, and suggest involvement of farnesoid X receptor activation pathways. |
|


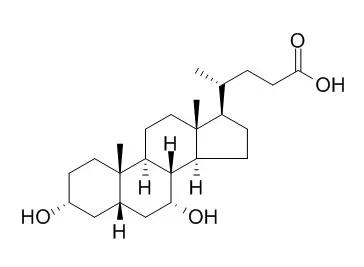



 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)