| Animal Research: |
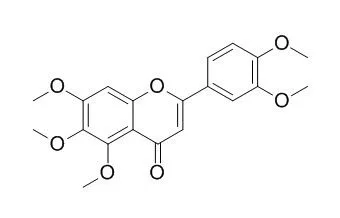
| Mol Nutr Food Res. 2012 Jun;56(6):945-56. | | In vitro and in vivo structure and activity relationship analysis of polymethoxylated flavonoids: identifying sinensetin as a novel antiangiogenesis agent.[Pubmed: 22707269] | Polymethoxylated flavonoids are present in citrus fruit in a range of chemical structures and abundance. These compounds have potential for anticarcinogenesis, antitumor, and cardiovascular protective activity, but the effect on angiogenesis has not been well studied.
METHODS AND RESULTS:
Human umbilical vein endothelial cells (HUVECs) in vitro and zebrafish (Danio rerio) in vivo models were used to screen and identify the antiangiogenesis activity of seven polymethoxylated flavonoids; namely, hesperetin, naringin, neohesperidin, nobiletin, scutellarein, scutellarein tetramethylether, and Sinensetin. Five, excluding naringin and neohesperidin, showed different degrees of potency of antiangiogenesis activity. Sinensetin, which had the most potent antiangiogenesis activity and the lowest toxicity, inhibited angiogenesis by inducing cell cycle arrest in the G0/G1 phase in HUVEC culture and downregulating the mRNA expressions of angiogenesis genes flt1, kdrl, and hras in zebrafish.
CONCLUSIONS:
The in vivo structure-activity relationship (SAR) analysis indicated that a flavonoid with a methoxylated group at the C3' position offers a stronger antiangiogenesis activity, whereas the absence of a methoxylated group at the C8 position offers lower lethal toxicity in addition to enhancing the antiangiogenesis activity. This study provides new insight into how modification of the chemical structure of polymethoxylated flavonoids affects this newly identified antiangiogenesis activity. |
|
| Structure Identification: |
| Food Chem., 2009, 113(1): 185-90. | | Sinensetin, rutin, 3'-hydroxy-5, 6, 7, 4'-tetramethoxyflavone and rosmarinic acid contents and antioxidative effect of the skin of apple fruit[Reference: WebLink] |
METHODS AND RESULTS:
A GC–MS method was developed for the separation and quantifiation of three flavones: Sinensetin (SEN), rutin (RU) and 3′-hydroxy-5, 6, 7, 4′-tetramethoxyflavone (TMF) and rosmarinic acid (RA), a caffeic acid derivative, in the skin of apple fruit collected from different local markets of Bangladesh. The results showed significant variation in the amount of these markers in methanolic extracts of skin samples from different markets of Bangladesh, even though the values were almost identical for most of the cases. A variation in antioxidant activities, ranging from 62.82 to 92.34%, and variations in total phenolics, ranging from 6.69 to 10.20 mg caffeic acid/g dry weight of the methanol extracts, were observed.
CONCLUSIONS:
Antioxidative potency of the methanolic extracts was comparable to that of pure quercetin and the synthetic antioxidant butylated hydroxylanisole (BHA). |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)