| Kinase Assay: |
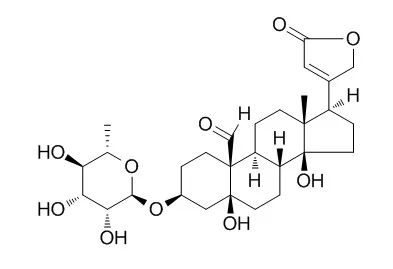
| Plos One, 2014, 9(3):e91094. | | Convallatoxin, a Dual Inducer of Autophagy and Apoptosis, Inhibits Angiogenesis In Vitro and In Vivo.[Reference: WebLink] | Autophagy and apoptosis are important processes that control cellular homeostasis and have been highlighted as promising targets for novel cancer therapies.
METHODS AND RESULTS:
Here, we identified Convallatoxin (CNT), isolated from Antiaris toxicaria, as a dual inducer of autophagy and apoptosis. CNT exerts cytotoxic effects on a number of cancer and normal cell lines and induces apoptosis by increasing caspase-3 and poly ADP ribose polymerase (PARP) cleavage. Moreover, dose- and time-dependent autophagic activity was detected in CNT-treated cells, and mammalian target of rapamycin (mTOR)/p70S6K signal pathway inhibition was observed. Notably, CNT inhibits human umbilical vein endothelial cell (HUVEC) growth and exerts anti-angiogenic activity in vitro and in vivo.
CONCLUSIONS:
Collectively, these results demonstrate that the naturally occurring compound, CNT, is a novel anti-angiogenic compound via dual inducing of autophagy and apoptosis. | | Molecular Genetics & Metabolism Reports, 2017, 13:42-45. | | The cardiac glycoside convallatoxin inhibits the growth of colorectal cancer cells in a p53-independent manner.[Reference: WebLink] | Cardiac glycosides are plant-derived molecules that have shown antiproliferative properties against cancer cells, though the mechanism of action is not completely understood.
METHODS AND RESULTS:
We show that one cardiac glycoside, Convallatoxin, presents antiproliferative effects against colorectal cancer cells in culture and that the resulting cell death is independent of the p53 tumor suppressor.
CONCLUSIONS:
Our data suggest that Convallatoxin may be useful in the treatment of cancers that harbor inactivating mutations in the p53 signaling pathway. |
|
| Cell Research: |
| Cell Death Discovery, 2017, 3:17009. | | Antitumor effects of naturally occurring cardiac glycosides convallatoxin and peruvoside on human ER+ and triple-negative breast cancers.[Reference: WebLink] | Breast cancer is second most prevalent cancer in women, and the second only to lung cancer in cancer-related deaths. It is a heterogeneous disease and has several subtypes based on the presence or absence of hormone receptors and/or human epidermal growth factor receptor 2 (HER2). Hormone receptor-positive and HER2-enriched cancers can be targeted using hormone and HER2-targeting therapies such as trastuzumab or lapatinib. However, triple-negative breast cancers (TNBCs) do not express any of the receptors and therefore are resistant to most targeted therapies, and cytotoxic chemotherapies are the only viable option available for the treatment of TNBCs. Recently, cardiac glycosides (CGs) have emerged as potential anticancer agents that impart their antiproliferative effect by targeting multiple pathways. In this study our aim was to evaluate anticancer effects of two naturally occurring CGs, Convallatoxin (CT) and Peruvoside (PS), on ER+ and TNBCs cells.
METHODS AND RESULTS:
CT and PS demonstrated dose- and time-dependent cytotoxic effect on MCF-7 cells, which was further supported by loss of colony formation on drug treatment. CT and PS arrested MCF-7 cells in the G0/G1 phase and reduced the viability of MCF-7-derived mammospheres (MMs). Interestingly, while CT and PS imparted cell death in TNBCs cells from both Caucasians (MDA-MB-231 cells) and African Americans (MDA-MB-468 cells) in a dose- and time-dependent manner, the drugs were much more potent in MDA-MB-468 as compared with TNBC MDA-MB-231 cells. Both drugs significantly inhibited migration and invasion of both MCF-7 and MDA-MB-468 cells. An assessment of intracellular pathways indicated that both drugs were able to modulate several key cellular pathways such as EMT, cell cycle, proliferation and cell death in both cell types.
CONCLUSIONS:
Our data suggest a promising role for CGs in breast cancer treatment specifically in targeting TNBCs derived from African Americans, and provides impetus for further investigation of the anticancer potential of this class of drugs. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)