| Structure Identification: |
| Journal of Natural Products,2004,44(4):475-7. | | (-)-Corlumine, A New Phthalideisoquinoline Alkaloid From Fumaria parviflora[Reference: WebLink] |
METHODS AND RESULTS:
Fumaria parviflora Lam. (Fumariaceae) has yielded the new phthalideisoquinoline alkaloid (-)-Corlumine (2) previously known only in its dextrorotatory form. Two known alkaloids not previously detected in F. parviflora are (+)-adlumidine (1) and (-)-cheilanthifoline (3).
CONCLUSIONS:
Phthalideisoquinoline alkaloids as isolated from plants show no pronounced stereochemical preference either at C-1 or at C-9. Most protoberberine alkaloids, however, possess the S- chirality at C-14. | | Spectrochim Acta A Mol Biomol Spectrosc. 2014 Jan 24;118:470-80. | | Quantum chemical and experimental studies on the structure and vibrational spectra of an alkaloid--Corlumine.[Pubmed: 24080578 ] |
METHODS AND RESULTS:
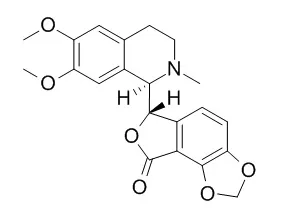
The study concentrates on an important natural product, phthalide isoquinoline alkaloid Corlumine (COR) [(6R)-6-[(1S)-1,2,3,4-Tetrahydro-6,7-dimethoxy-2-methylisoquinolin-1-yl] furo [3,4-e]-1,3-benzodioxol-8(6H)-one] well known to exhibit spasmolytic and GABA antagonist activity. It was fully characterized by a variety of experimental methods including vibrational spectroscopy (IR and Raman), thermal analysis (DSC), UV and SEM. For a better interpretation and analysis of the results quantum chemical calculations employing DFT were also performed. TD-DFT was employed to elucidate electronic properties for both gaseous and solvent environment using IEF-PCM model. Graphical representation of HOMO and LUMO would provide a valuable insight into the nature of reactivity and some of the structural and physical properties of the title molecule.
CONCLUSIONS:
The structure-activity relationship have been interpreted by mapping electrostatic potential surface (MEP), which is valuable information for the quality control of medicines and drug-receptor interactions. Stability of the molecule arising from hyper conjugative interactions, charge delocalisation has been analyzed using natural bond orbital (NBO) analysis. Computation of thermodynamical properties would help to have a deep insight into the molecule for further applications. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)