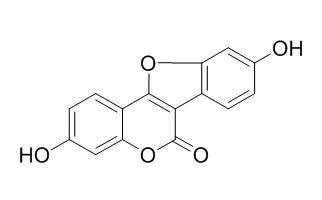
| Description: |
Coumestrol is a novel inducer of mitochondrial biogenesis through the activation of Sirt1, it suppresses the accumulation of HIF-1α via suppression of SPHK1 pathway in hypoxic PC-3 cells. Coumestrol can function by inhibiting oncogenic disease, at least in part, through CKII inhibition-mediated cellular senescence. Coumestrol treatment is effective in preventing neuronal loss in all times of administration as well as able to rescue the Na+, K+ -ATPase activity, suggesting its potential benefits for either prevention or therapeutics use against cerebral ischemia in males. |
| In vitro: |
| J Agric Food Chem. 2014 May 14;62(19):4298-305. | | Coumestrol induces mitochondrial biogenesis by activating Sirt1 in cultured skeletal muscle cells.[Pubmed: 24712520 ] | The mitochondrion is a central organelle in cellular energy homeostasis; thus, reduced mitochondrial activity has been associated with aging and metabolic disorders. This paper provides biological evidence that Coumestrol, which is a natural isoflavone, activates mitochondrial biogenesis.
METHODS AND RESULTS:
In cultured myocytes, Coumestrol activated the silent information regulator two ortholog 1 (Sirt1) through the elevation of the intracellular NAD(+)/NADH ratio. Coumestrol also increased the mitochondrial contents and induced the expression of key proteins in the mitochondrial electron transfer chain in cultured myocytes. A Sirt1 inhibitor and Sirt1-targeting siRNAs abolished the effect of Coumestrol on mitochondrial biogenesis. Similar to an increase in mitochondrial content, Coumestrol improved myocyte function with increased ATP concentration.
CONCLUSIONS:
Taken together, the data suggest that Coumestrol is a novel inducer of mitochondrial biogenesis through the activation of Sirt1. | | J Endocrinol . 2016 Mar;228(3):149-60. | | Coumestrol suppresses proliferation of ES2 human epithelial ovarian cancer cells[Pubmed: 26698565] | | Abstract
Coumestrol, which is predominantly found in soybean products as a phytoestrogen, has cancer preventive activities in estrogen-responsive carcinomas. However, effects and molecular targets of Coumestrol have not been reported for epithelial ovarian cancer (EOC). In the present study, we demonstrated that Coumestrol inhibited viability and invasion and induced apoptosis of ES2 (clear cell-/serous carcinoma origin) cells. In addition, immunoreactive PCNA and ERBB2, markers of proliferation of ovarian carcinoma, were attenuated in their expression in Coumestrol-induced death of ES2 cells. Phosphorylation of AKT, p70S6K, ERK1/2, JNK1/2, and p90RSK was inactivated by Coumestrol treatment in a dose- and time-dependent manner as determined in western blot analyses. Moreover, PI3K inhibitors enhanced effects of Coumestrol to decrease phosphorylation of AKT, p70S6K, S6, and ERK1/2. Furthermore, Coumestrol has strong cancer preventive effects as compared to other conventional chemotherapeutics on proliferation of ES2 cells. In conclusion, Coumestrol exerts chemotherapeutic effects via PI3K and ERK1/2 MAPK pathways and is a potentially novel treatment regimen with enhanced chemoprevention activities against progression of EOC.
Keywords: clear cell carcinoma; Coumestrol; ovary; reproduction; reproductive tract. | | Food Chem Toxicol . 2017 Jan;99:149-161. | | Cytotoxic activity of soy phytoestrogen coumestrol against human breast cancer MCF-7 cells: Insights into the molecular mechanism[Pubmed: 27913286] | | Abstract
Coumestrol is a phytoestrogen present in soybean products and recognized as potential cancer therapeutic agent against breast cancer. However, the clear molecular mechanism of anticancer-activity of Coumestrol in breast carcinoma has not been reported. It is well established that copper levels are elevated in different malignancies. Therefore, the objective of this study was to investigate the copper-dependent cytotoxic action of Coumestrol in human breast cancer MCF-7 cells. Results showed that Coumestrol inhibited proliferation and induced apoptosis in MCF-7 cells, which was prevented by copper chelator neocuproine and ROS scavengers. Coumestrol treatment induced ROS generation coupled to DNA fragmentation, up-regulation of p53/p21, cell cycle arrest at G1/S phase, mitochondrial membrane depolarization and caspases 9/3 activation. All these effects were suppressed by ROS scavengers and neocuproine. These results suggest that Coumestrol targets elevated copper for redox cycling to generate ROS leading to DNA fragmentation. DNA damage leads to p53 up-regulation which directs the cell cycle arrest at G1/S phase and promotes caspase-dependent apoptosis of MCF-7 cells. In conclusion, copper targeted ROS-mediated p53-dependent mechanism better explains the cytotoxic action of Coumestrol in MCF-7 cells. Thus, targeting elevated copper levels might be a potential therapeutic strategy for selective cytotoxic action against malignant cells.
Keywords: Apoptosis; Breast cancer; Copper; Coumestrol; DNA damage; ROS. |
|
| In vivo: |
| Biochem Pharmacol. 2015 Jan 1;93(1):42-8. | | Coumestrol inhibits carotid sinus baroreceptor activity by cAMP/PKA dependent nitric oxide release in anesthetized male rats.[Pubmed: 25449602] | Phytoestrogens could offer multiple beneficial effects on the cardiovascular system. Here, we have examined the effects of Coumestrol (CMT) on carotid baroreceptors activity (CBA) and the possible mechanisms in male rats. The functional parameters of carotid baroreceptors were measured by recording sinus nerve afferent discharge in anesthetized male rats with perfused isolated carotid sinus.
METHODS AND RESULTS:
The levels of protein expression were determined by using ELISA and Western blotting. CMT (1 to 100μmolL(-1)) inhibited CBA, which shifted the functional curve of the carotid baroreceptor to the right and downward, with a marked decrease in the peak slope and the peak integral value of carotid sinus nerve discharge in a concentration dependent manner. These effects were not blocked by a specific estrogen receptor antagonist ICI 182,780, but were completely abolished by nitric oxide (NO) synthase inhibitor l-NAME (N(G)-nitro-l-arginine methyl ester). Furthermore, a NO donor, SIN-1(3-morpholion-sydnon-imine), could potentiate these inhibitory effects of CMT. CMT stimulated the phosphorylation of Ser(1176)-eNOS (endothelial nitric oxide synthase) in a dose-dependent manner in carotid bifurcation tissue over a perfusion period of 15min.
CONCLUSIONS:
The rapid activation of eNOS by CMT was blocked by a highly selective PKA (protein kinase A) inhibitor H89. In addition, inhibition of PI3K (phosphatidylinositol-3-kinase) and ERK (extracellular signal-regulated kinase) pathways had no effect on eNOS activation by CMT. CMT inhibited CBA via eNOS activation and NO synthesis. These effects were mediated by the cAMP/PKA pathway and were unrelated to the estrogenic effect. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)