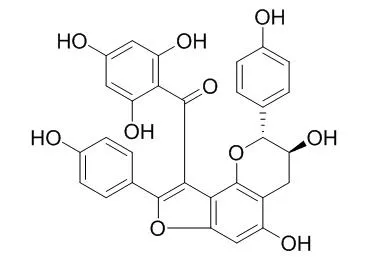
| Description: |
Daphnodorin B has antifungal, antimitotic and anti-HIV-1 activity, it induced morphological deformation of P. oryzae mycelia with MMDC values of 73.7 +/- 1.6 microM, it showed moderate activity against microtubule polymerization with IC50 values of 142 +/- 2 microM in vitro, and it moderately active against HIV-1 in vitro. Daphnodorin B shows inhibitory effects of human chymase. Daphnodorin B inhibited tumor growth and metastasis by protecting host immunocyte viability and proliferation potential, and selectively inhibiting tumor cell proliferation. |
| In vitro: |
| Phytochemistry. 2014 Oct;106:61-68. | | Phytotoxic flavonoids from roots of Stellera chamaejasme L. (Thymelaeaceae).[Pubmed: 25096753 ] | Allelopathy, the negative effect on plants of chemicals released to the surroundings by a neighboring plant, is an important factor which contributes to the spread of some weeds in plant communities. In this field, Stellera chamaejasme L. (Thymelaeaceae) is one of the most toxic and ecologically-threatening weeds in some of the grasslands of north and west China.
METHODS AND RESULTS:
Bioassay-guided fractionation of root extracts of this plant led to the isolation of eight flavonoids 1-8, whose structures were elucidated by spectroscopic analysis. All compounds obtained, except 7-methoxylneochaejasmin A (4) and (+)-epiafzelechin (5), showed strong phytotoxic activity against Arabidopsis thaliana seedlings. Seedling growth was reduced by neochamaejasmin B (1), mesoneochamaejasmin A (2), chamaejasmenin C (3), genkwanol A (6), Daphnodorin B (7) and dihydroDaphnodorin B (8) with IC50 values of 6.9, 12.1, 43.2, 74.8, 7.1 and 27.3μg/mL, respectively, and all of these compounds disrupted root development. Endogenous auxin levels at the root tips of the A. thaliana DR5::GUS transgenic line were largely reduced by compounds 1, 2 and 6-8, and were increased by compound 4. Moreover, the inhibition rate of A. thaliana auxin transport mutants pin2 and aux1-7 by compounds 1-8 were all lower than the wild type (Col-0). The influence of these compounds on endogenous auxin distribution is thus proposed as a critical factor for the phytotoxic effect. Compounds 1, 2, 4 and 8 were found in soils associated with S. chamaejasme, and these flavonoids also showed phytotoxicity to Clinelymus nutans L., an associated weed of S. chamaejasme.
CONCLUSIONS:
These results indicated that some phytotoxic compounds from roots of S. chamaejasme may be involved in the potential allelopathic behavior of this widespread weed. | | Planta Med. 2000 Aug;66(6):564-7. | | Antifungal, antimitotic and anti-HIV-1 agents from the roots of Wikstroemia indica.[Pubmed: 10985087] |
METHODS AND RESULTS:
With guidance of Pyricularia oryzae bioassay, daphnoretin (1), (+)-nortrachelogenin (2), genkwanol A (3), wikstrol A (4), wikstrol B (5) and Daphnodorin B (6) were isolated from the roots of Wikstroemia indica. Compounds 1-6 induced morphological deformation of P. oryzae mycelia with MMDC values of 68.4 +/- 1.3, 31.3 +/- 1.8, 45.8 +/- 0.5, 70.1 +/- 2.4, 52.3 +/- 0.9 and 73.7 +/- 1.6 microM, respectively. Compounds 3-6 showed moderate activity against microtubule polymerization with IC50 values of 112 +/- 4, 131 +/- 3, 184 +/- 6 and 142 +/- 2 microM in vitro, respectively.
Compounds 2, 3, 5 and 6 were moderately active against HIV-1 in vitro.
CONCLUSIONS:
The findings of bioactivity of 1-6 support the antifungus, antimitosis and anti-HIV-1 uses for W. indica roots. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)