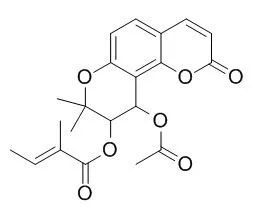
| Description: |
Praeruptorin A exerts neuroprotective, anti-osteoclastogenic, anti-inflammatory, distinct relaxant effects, it is beneficial to facilitate nestin expression in myocarditis,and suitable in treatment of early myocarditis. Praeruptorin A can significantly up-regulate UGT1A1 expression in HepG2 cells partially via the CAR-mediated pathway. Praeruptorin A inhibited p38/Akt-c-Fos-NFATc1 signaling and PLCγ-independent Ca2+ oscillation. |
| Targets: |
Calcium Channel | p38MAPK | Akt | NO | IL Receptor | TNF-α | NF-kB | IkB | Bcl-2/Bax | SOD | IKK |
| In vitro: |
| J Nat Prod. 2015 Apr 24;78(4):776-82. | | Praeruptorin a inhibits in vitro migration of preosteoclasts and in vivo bone erosion, possibly due to its potential to target calmodulin.[Pubmed: 25734761] | Excessive activity and/or increased number of osteoclasts lead to bone resorption-related disorders. Here, we investigated the potential of Praeruptorin A to inhibit migration/fusion of preosteoclasts in vitro and bone erosion in vivo.
METHODS AND RESULTS:
Praeruptorin A inhibited the RANKL-induced migration/fusion of preosteoclasts accompanied by the nuclear translocation of NFATc1, a master regulator of osteoclast differentiation. Antimigration/fusion activity of Praeruptorin A was also confirmed by evaluating the mRNA expression of fusion-mediating molecules. In silico binding studies and several biochemical assays further revealed the potential of Praeruptorin A to bind with Ca(2+)/calmodulin and inhibit its downstream signaling pathways, including the Ca(2+)/calmodulin-CaMKIV-CREB and Ca(2+)/calmodulin-calcineurin signaling axis responsible for controlling NFATc1. In vivo application of Praeruptorin A significantly reduced lipopolysaccharide-induced bone erosion, indicating its possible use to treat bone resorption-related disorders.
CONCLUSIONS:
In conclusion, Praeruptorin A has the potential to inhibit migration/fusion of preosteoclasts in vitro and bone erosion in vivo by targeting calmodulin and inhibiting the Ca(2+)/calmodulin-CaMKIV-CREB-NFATc1 and/or Ca(2+)/calmodulin-calcineurin-NFATc1 signaling axis. | | PLoS One. 2014 Feb 21;9(2):e88974. | | Anti-osteoclastogenic activity of praeruptorin A via inhibition of p38/Akt-c-Fos-NFATc1 signaling and PLCγ-independent Ca2+ oscillation.[Pubmed: 24586466] | A decrease of bone mass is a major risk factor for fracture. Several natural products have traditionally been used as herbal medicines to prevent and/or treat bone disorders including osteoporosis. Praeruptorin A is isolated from the dry root extract of Peucedanum praeruptorum Dunn and has several biological activities, but its anti-osteoporotic activity has not been studied yet.
METHODS AND RESULTS:
The effect of Praeruptorin A on the differentiation of bone marrow-derived macrophages into osteoclasts was examined by phenotype assay and confirmed by real-time PCR and immunoblotting. The involvement of NFATc1 in the anti-osteoclastogenic action of Praeruptorin A was evaluated by its lentiviral ectopic expression. Intracellular Ca(2+) levels were also measured.
Praeruptorin A inhibited the RANKL-stimulated osteoclast differentiation accompanied by inhibition of p38 and Akt signaling, which could be the reason for Praeruptorin A-downregulated expression levels of c-Fos and NFATc1, transcription factors that regulate osteoclast-specific genes, as well as osteoclast fusion-related molecules. The anti-osteoclastogenic effect of Praeruptorin A was rescued by overexpression of NFATc1. Praeruptorin A strongly prevented the RANKL-induced Ca(2+) oscillation without any changes in the phosphorylation of PLCγ.
CONCLUSIONS:
Praeruptorin A could exhibit its anti-osteoclastogenic activity by inhibiting p38/Akt-c-Fos-NFATc1 signaling and PLCγ-independent Ca(2+) oscillation. | | Phytother Res. 2011 Apr;25(4):550-6. | | Praeruptorin A inhibits lipopolysaccharide-induced inflammatory response in murine macrophages through inhibition of NF-κB pathway activation.[Pubmed: 20842678 ] | Praeruptorin A (PA) is a pyranocoumarin compound isolated from the dried root of Peucedanum praeruptorum Dunn (Umbelliferae). However, the antiinflammatory effect of PA has not been reported.
METHODS AND RESULTS:
The present study investigated the antiinflammatory effect of PA in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophage cells. PA significantly inhibited the LPS-induced production of nitric oxide (NO), interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α). The mRNA and protein expressions of inducible nitric oxide synthase (iNOS), IL-1β and TNF-α were also suppressed by this compound. Further study showed that PA decreased the cytoplasmic loss of inhibitor κB-α (IκB-α) protein and inhibited the translocation of NF-κB from cytoplasm to nucleus.
CONCLUSIONS:
Taken together, the results suggest that PA may exert antiinflammatory effects in vitro in LPS-stimulated RAW 264.7 macrophages through inhibition of NF-κB signal pathway activation. |
|
| In vivo: |
| Pharmacology & Clinics of Chinese Materia Medica, 2007, 23(3):21-3. | | Praeruptorin A upregulates expression of nestin in experimental autoimmune myocarditis of rats[Reference: WebLink] | To investigate the influence of dl-Praeruptorin A(Pd-Ia) on expression of intermediate filament protein nestin and its cardioprotective actions in myocarditis.[WT5"HZ]
METHODS AND RESULTS:
Effect of Pd-Ia on expression of nestin was evaluated in rats that had recovered from experimental autoimmune myocarditis(EAM).Wistar rats were divided into 5 groups,including group sham,group natural saline,group solvent control(polyethylene glycol),group Pd-Ia 1.0 mg/kg,and group positive control(verapamil0.5 mg/kg),respectively.Thereafter,all animals were immunized with pig cardiac myosin on day 0.Drugs were administered twice through abdominal cavity on day 20 and day 21,respectively.Four hours after second administration,rats are executed.Expression of nestin in myocardium was detected by Western blot analysis.Heart weight/body weight ratio,myocardial enzyme spectrum,and histopathology were measured simultaneously. Results:Pd-Ia upregulated expression of nestin and relieved myocardial injury in EAM of rats.Values of relative optic density in Pd-Ia(1.0 mg/kg) group were increased from 37.5 ± 3 to 61 ± 4(P0.05,vs solvent,n=5).
CONCLUSIONS:
[WT5"BZ] Pd-Ia was beneficial to facilitate nestin expression in myocarditis,and suitable in treatment of early myocarditis. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)