Underground parts of three Valeriana species, namely V. officinalis L. s.l., V. wallichii DC. (V. jatamansi Jones), and V. edulis Nutt. ex Torr & Gray ssp. procera (H.B.K.) F. G. Meyer (V. mexicana DC.), are used in phytotherapy because of their mild sedative properties.
METHODS AND RESULTS:
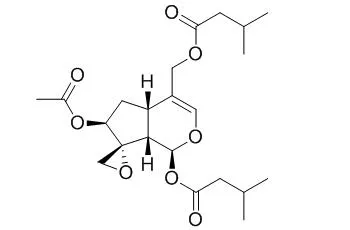
Characteristic constituents of these species, which are regarded also as the active principles, were tested for cytotoxicity against GLC(4), a human small-cell lung cancer cell line, and against COLO 320, a human colorectal cancer cell line, using the microculture tetrazolium (MTT) assay. Valepotriates of the diene type (valtrate, isovaltrate and acevaltrate) displayed the highest cytotoxicity, with IC50 values of 1-6 μM, following continuous incubation. The monoene type valepotriates (Didrovaltrate and isovaleroxyhydroxyDidrovaltrate) were 2- to 3-fold less toxic. Baldrinal and homobaldrinal, decomposition products of valepotriates, were 10- to 30-fold less toxic than their parent compounds. Isovaltral had a higher cytotoxicity than its parent compound isovaltrate. Valerenic acids (valerenic acid, acetoxyvalerenic acid, hydroxyvalerenic acid and methyl valerenate), which are characteristic for V. officinalis, had a low toxicity with IC(50) values between 100 and 200 μM. Freshly prepared and stored tinctures, prepared from roots and rhizomes of the three valerian species, were analysed for valepotriates, baldrinals and valerenic acids, and also tested for cytotoxicity.
CONCLUSIONS:
There was a clear relationship between the valepotriate contents of the freshly prepared tinctures and their toxicity. Upon storage, valepotriates decomposed, which was reflected in a significant reduction of the cytotoxic effect. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)