| Structure Identification: |
| The Alkaloids: Chemistry and Physiology Volume 11, 1968, Pages 73–77 | | Chapter 3 The 2,2′-Indolylquinuclidine Alkaloids[Reference: WebLink] |
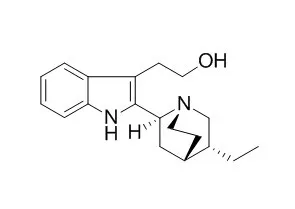
The group of 2,2′-indolylquinuclidine alkaloids has a narrow distribution and only two members have been discovered in the past few years. This chapter focuses on accepted stereochemistry, although an unequivocal proof is still required for C-3 in cinchonamine.
METHODS AND RESULTS:
The course of the acetylation of cinchonamine has been restudied using the parent base, Dihydrocinchonamine and O-tritylcinchonamine. Many of the compounds formed in this investigation were amorphous, but were satisfactorily characterized via thin-layer chromatography and mass spectrometry, along with UV- and IR-measurements. At low temperatures (dry ice-acetone), cinchonamine yields O-acetylcinchonamine. Dihydrocinchonamine behaves analogously upon treatment with acetic anhydride, although there are difficulties because of the amorphous nature of the products.
CONCLUSIONS:
Cinchophyllamine contains two 5-methoxyindole nuclei with unsubstituted nitrogen atoms, a vinyl group, and two basic nitrogens of which one is tertiary and the other secondary. A reinvestigation of the alkaloid content of the leaves of Cinchona legeriana Moens gives, besides quinamine, two new bases analyzing for C31H36N4O2—cinchophyllamine and isocinchophyllamine. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)