| In vitro: |
| Nat Prod Res. 2016;30(4):433-7. | | Cytotoxicity and Synergistic Effect of the Constituents from Roots of Aglaia odorata (Meliaceae).[Pubmed: 25742723] |
METHODS AND RESULTS:
Twelve compounds were isolated from the roots of Aglaia odorata. Their structures were established on the basis of NMR and MS data as rocaglaol (1), rocaglamide (2), eichlerialactone (3), sapelins A (4), isofouquierone (5), Eichlerianic acid (6), shoreic acid (7), agladupol E (8), 3-epimeliantriol (9), cleomiscosins B (10), 2β,3β-dihydroxy-5α-pregnane-16-one (11) and β-d-glucopyranos-1-yl N-methylpyrrole-2-carboxylate (12).
CONCLUSIONS:
Among them, compounds 1 and 2 showed significant cytotoxicity against human cancer cell (HL-60, SMMC-7721, A-549, MCF-7 and SW480) with IC50 values of 0.007-0.095 μM, while compounds 3-5 and 10 and 11 showed moderate to no cytotoxicity (IC50 0.43 to values >40 μM). Compound 6 showed only weak cytotoxicity (IC50 6.87 to >40 μM) and its epmier 7 was completely inactivite (IC50>40 μM) in the assay. However, potent synergistic effect was observed when the molar ratio of 6 to 7 is between 4:1 and 1:1. | | J Nat Prod. 1987 Jul-Aug;50(4):706-13. | | In vitro antiviral activity of dammar resin triterpenoids.[Pubmed: 2828553] |
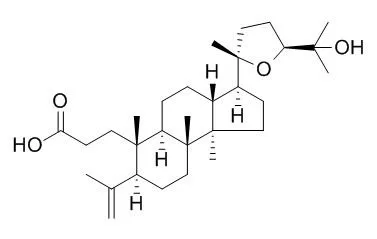
METHODS AND RESULTS:
Nine triterpenes with antiviral activity against Herpes simplex virus types I and II in vitro were isolated from dammar resin. Each compound caused a significant reduction in viral cytopathic effect when Vero cells were exposed continuously to 1-10 micrograms/ml of compound for 48 h after viral challenge.
CONCLUSIONS:
The triterpenes were identified as dammaradienol [1], dammarenediol-II [2], hydroxydammarenone-I [3], ursonic acid [5], hydroxyhopanone [11], dammarenolic acid [15], shoreic acid [16], Eichlerianic acid [17], and a novel compound, hydroxyoleanonic lactone [7], on the basis of their chromatographic, spectroscopic, and physical properties. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)