| In vitro: |
| J Agric Food Chem. 2005 Jun 1;53(11):4593-8. | | Inhibitory effects of oolong tea polyphenols on pancreatic lipase in vitro.[Pubmed: 15913331] | Fifty-four polyphenols isolated from tea leaves were evaluated for their inhibitory activities against pancreatic lipase, the key enzyme of lipid absorption in the gut.
METHODS AND RESULTS:
(-)-Epigallocatechin 3-O-gallate (EGCG), which is one of major polyphenols in green tea, showed lipase inhibition with an IC50 of 0.349 microM. Moreover, flavan-3-ol digallate esters, such as (-)-epigallocatechin-3,5-digallate, showed higher activities of inhibition on lipase with an IC50 of 0.098 microM. On the other hand, nonesterified flavan-3-ols, such as (+)-catechin, (-)-epicatechin, (+)-gallocatechin, and (-)-epigallocatechin, showed zero and/or the lowest activities against pancreatic lipase (IC50 > 20 microM). These data suggested that the presence of galloyl moieties within the structure was required for enhancement of pancreatic lipase inhibition. It is well-known that flavan-3-ols are polymerized by polyphenol oxidase and/or heating in a manufacturing process of oolong tea. Oolonghomobisflavans A and B and oolongtheanin 3'-O-gallate, which are typical in oolong tea leaves, showed strong inhibitory activities with IC50 values of 0.048, 0.108, and 0.068 microM, respectively, even higher than that of EGCG. The oolong tea polymerized polyphenols (OTPP) were prepared for the assay from oolong tea extract, from which the preparation effectively subtracted the zero and/or less-active monomeric flavan-3-ols by preparative high-performance liquid chromatography. The weight-average molecular weight (Mw) and number-average molecular-weight (Mn) values of OTPP were 2017 and 903, respectively, by using gel permeation choromatography. OTPP showed a 5-fold stronger inhibition against pancreatic lipase (IC50 = 0.28 microg/mL) by comparison with that of the tannase-treated OTPP (IC50 = 1.38 microg/mL).
CONCLUSIONS:
These data suggested that the presence of galloyl moieties within their chemical structures and/or the polymerization of flavan-3-ols were required for enhancement of pancreatic lipase inhibition. | | Nat Prod Res . 2018 Feb;32(4):453-456. | | Antioxidant phenolic compounds from the rhizomes of Astilbe rivularis[Pubmed: 28361551] | | Abstract
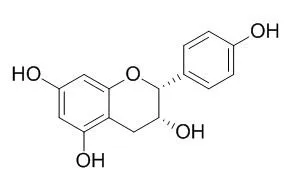
The rhizomes of Astilbe rivularis, commonly known as 'Thulo Okhati' are widely used in Nepal as tonic for uterine and menstrual disorders. In our preliminary study, the 70% MeOH extract of the rhizomes showed potent antioxidant activity. Hence, present study was aimed for the isolation of potent antioxidant constituents. Bergenin (1), 11-O-galloylbergenin (2), (+)-catechin (3), (-)-catechin (4), (-)-afzelechin (5), (-)-Epiafzelechin (6) and 2-(β-D-glucopyranosyloxy)-4-hydroxylbenzenacetonitrile (7) were isolated from the rhizomes. Structures of these compounds were elucidated on the basis of spectroscopic methods. All these isolated compounds were evaluated for their in vitro antioxidant activity by 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay. 11-O-Galloylbergenin (2), (+)-catechin (3), (-)-catechin (4), (-)-afzelechin (5) and (-)-Epiafzelechin (6) showed potent antioxidant activity.
Keywords: Astilbe rivularis; Thulo Okhati; antioxidant activity; bergenin. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)