| J Org Chem. 2014 Sep 5;79(17):8481-5. |
| Synthesis of the erythrina alkaloid erysotramidine.[Pubmed: 25140810] |
METHODS AND RESULTS:
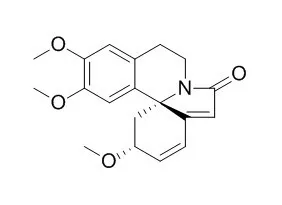
A concise synthesis of Erysotramidine (an alkaloid belonging to the erythrina family) was achieved starting with an inexpensive phenol and amine derivative. The synthesis is based on oxidative phenol dearomatizations mediated by a hypervalent iodine reagent and includes a novel route to a key indolinone moiety. |
| J Org Chem. 2015 Feb 6;80(3):1957-63. |
| Heck cyclization strategy for preparation of erythrinan alkaloids: asymmetric synthesis of unnatural (-)-erysotramidine from L-tartaric acid.[Pubmed: 25569446] |
METHODS AND RESULTS:
With an imide derived from L-tartaric acid as the starting material, ent-Erysotramidine was synthesized for the first time. The synthesis features the use of the enantiopure synthon, prepared in a set of highly stereoselective reactions, including N-acyliminium cyclization, dihydrofuranyl ring formation via silver-catalyzed intramolecular alcohol addition to acetylene, and vinyl ether catalytic hydrogen reduction.
CONCLUSIONS:
The crucial step of the synthesis, assembly of ring A, was achieved by using Heck cyclization of (Z)-iodoolefin. |
| Org Lett. 2009 Nov 19;11(22):5230-3. |
| Efficient formal total synthesis of the erythrina alkaloid (+)-erysotramidine, using a domino process.[Pubmed: 19860384] |
METHODS AND RESULTS:
A domino process consisting of an amidation, spirocyclization, and formation of an iminium ion and electrophilic aromatic substitution of a phenylethylamine and a ketoester leads to the spirocyclic skeleton of (+)-Erysotramidine, which can be further transformed into the natural alkaloid. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)