| Description: |
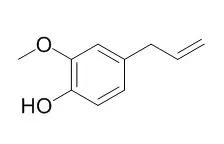
Eugenol is an essential oil found in cloves with herbicide,sedative,analgesic,antibacterial,anthelmintic,anti-inflammatory, cancer chemopreventive and antioxidant activities. Eugenol is shown to inhibit lipid peroxidation, the mRNA expression of COX-2, but not COX-1 and inhibit the GABAA current in trigeminal ganglion neurons. It could be developed as therapeutic agent against diseases with excessive osteoclast activity. |
| In vitro: |
| PLoS One. 2015 Jan 30;10(1):e0117316. | | Eugenol inhibits the GABAA current in trigeminal ganglion neurons.[Pubmed: 25635877] | Eugenol has sedative, antioxidant, anti-inflammatory, and analgesic effects, but also serves as an irritant through the regulation of a different set of ion channels. Activation of gamma aminobutyric acid (GABA) receptors on sensory neurons leads to the stabilization of neuronal excitability but contributes to formalin-induced inflammatory pain.
METHODS AND RESULTS:
In this study, we examined the effect of Eugenol on the GABA-induced current in rat trigeminal ganglia (TG) neurons and in human embryonic kidney (HEK) 293 cells expressing the GABAA receptor α1β2γ2 subtype using the whole-cell patch clamp technique. RT-PCR and Western blot analysis were used to confirm the expression of GABAA receptor γ2 subunit mRNA and protein in the TG and hippocampus. Eugenol decreased the amplitude ratio of the GABA-induced current to 27.5 ± 3.2% (p < 0.05) in TG neurons, which recovered after a 3-min washout. In HEK 293 cells expressing the α1β2γ2 subtype, Eugenol inhibited GABA-induced currents in a dose-dependent manner. Application of Eugenol also decreased the GABA response in the presence of a G-protein blocker. Eugenol pretreatment with different concentrations of GABA resulted in similar inhibition of the GABA-induced current in a non-competitive manner. In conclusion, Eugenol inhibits the GABA-induced current in TG neurons and HEK 293 cells expressing the GABAA receptor in a reversible, dose-dependent, and non-competitive manner, but not via the G-protein pathway.
CONCLUSIONS:
We suggest that the GABAA receptor could be a molecular target for Eugenol in the modulation of nociceptive information. | | Pestic Biochem Physiol. 2015 Feb;118:64-70. | | Eugenol-inhibited root growth in Avena fatua involves ROS-mediated oxidative damage.[Pubmed: 25752432] | Plant essential oils and their constituent monoterpenes are widely known plant growth retardants but their mechanism of action is not well understood. We explored the mechanism of phytotoxicity of Eugenol, a monoterpenoid alcohol, proposed as a natural herbicide.
METHODS AND RESULTS:
Eugenol (100-1000 μM) retarded the germination of Avena fatua and strongly inhibited its root growth compared to the coleoptile growth. We further investigated the underlying physiological and biochemical alterations leading to the root growth inhibition. Eugenol induced the generation of reactive oxygen species (ROS) leading to oxidative stress and membrane damage in the root tissue. ROS generation measured in terms of hydrogen peroxide, superoxide anion and hydroxyl radical content increased significantly in the range of 24 to 144, 21 to 91, 46 to 173% over the control at 100 to 1000 μM Eugenol, respectively. The disruption in membrane integrity was indicated by 25 to 125% increase in malondialdehyde (lipid peroxidation byproduct), and decreased conjugated diene content (~10 to 41%). The electrolyte leakage suggesting membrane damage increased both under light as well as dark conditions measured over a period from 0 to 30 h. In defense to the oxidative damage due to Eugenol, a significant upregulation in the ROS-scavenging antioxidant enzyme machinery was observed. The activities of superoxide dismutases, catalases, ascorbate peroxidases, guaiacol peroxidases and glutathione reductases were elevated by ~1.5 to 2.8, 2 to 4.3, 1.9 to 5.0, 1.4 to 3.9, 2.5 to 5.5 times, respectively, in response to 100 to 1000 μM Eugenol.
CONCLUSIONS:
The study concludes that Eugenol inhibits early root growth through ROS-mediated oxidative damage, despite an activation of the antioxidant enzyme machinery. | | Life Sci. 2003 Jun 6;73(3):337-48. | | Eugenol suppresses cyclooxygenase-2 expression in lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells.[Pubmed: 12757841] | Inducible cyclooxygenase (COX-2) has been implicated in the processes of inflammation and carcinogenesis. Thus, the potential COX-2 inhibitors have been considered as anti-inflammatory or cancer chemopreventive agents.
METHODS AND RESULTS:
In this study, the methanolic extract of the cortex of Eugenia caryophyllata Thunberg (Myrtaceae) was found to potently inhibit the prostaglandin E(2) production in lipopolysaccharide (LPS)-activated mouse macrophage RAW264.7 cells (98.3% inhibition at the test concentration of 10 microg/ml). Further, hexane-soluble layer was the most active partition compared to ethyl acetate, n-butanol, and water-soluble parts. By bioassay-guided fractionation of hexane-soluble partition, Eugenol was isolated and exhibited a significant inhibition of PGE(2) production (IC(50) = 0.37 microM). In addition, Eugenol suppressed the cyclooxygenase-2 (COX-2) gene expression in LPS-stimulated mouse macrophage cells. On the line of COX-2 playing an important role in colon carcinogenesis further study was designed to investigate the effect of Eugenol on the growth and COX-2 expression in HT-29 human colon cancer cells. Eugenol inhibited the proliferation of HT-29 cells and the mRNA expression of COX-2, but not COX-1.
CONCLUSIONS:
This result suggests that Eugenol might be a plausible lead candidate for further developing the COX-2 inhibitor as an anti-inflammatory or cancer chemopreventive agent. | | J Ethnopharmacol. 2010 Jul 6;130(1):107-15. | | Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane.[Pubmed: 20435121 ] | To evaluate the antibacterial activity of Eugenol and its mechanism of bactericidal action against Salmonella typhi.
METHODS AND RESULTS:
The antibacterial activity was checked by disc-diffusion method, MIC, MBC, time course assay and pH sensitivity assay. The chemo-attractant property of Eugenol was verified by chemotaxis assay. The mode of action of Eugenol was determined by crystal violet assay, measurement of release of 260 nm absorbing material, SDS-PAGE, FT-IR spectroscopy, AFM and SEM.
Treatment with Eugenol at their MIC (0.0125%) and MBC (0.025%) reduced the viability and resulted in complete inhibition of the organism. Eugenol inactivated Salmonella typhi within 60 min exposure. The chemo-attractant property of Eugenol combined with the observed high antibacterial activity at alkaline pH favors the fact that the compound can work more efficiently when given in vivo. Eugenol increased the permeability of the membrane, as evidenced by crystal violet assay. The measurement of release of 260 nm absorbing intracellular materials, SDS-PAGE, SEM and AFM analysis confirmed the disruptive action of Eugenol on cytoplasmic membrane. The deformation of macromolecules in the membrane, upon treatment with Eugenol was verified by FT-IR spectroscopy.
CONCLUSIONS:
The results suggest that the antibacterial activity of Eugenol against Salmonella typhi is due to the interaction of Eugenol on bacterial cell membrane. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)