| Structure Identification: |
| J Nat Prod. 2004 Sep;67(9):1470-5. | | Cytotoxic sesquiterpene lactones from Eupatorium lindleyanum.[Pubmed: 15387644] |
METHODS AND RESULTS:
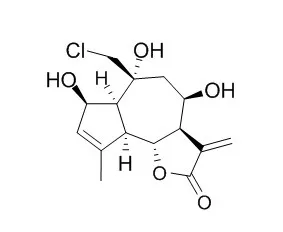
Ten new guaiane type sesquiterpene lactones, namely, Eupalinilide A, Eupalinilide B , Eupalinilide C, Eupalinilide D, Eupalinilide E, Eupalinilide F, Eupalinilide G, Eupalinilide H, Eupalinilide I, Eupalinilide J (1-10), as well as nine known compounds, eupachinilide C (11), eupachifolin D (12), eupachinilide E (13), 2alpha-hydroxyeupatolide (14), 3-deacetyleupalinin A (15), heliangine (16), 8beta-tigloyloxy-2,3-seco-6betaH,7alphaH-helianga-4Z,11(13)-diene-3,10beta;6, 12-diolid-2-oic acid (17), 8beta-(4'-hydroxytigloyloxy)-3beta,14-dihydroxy-6betaH,7alphaH-germacra-1(10)Z,4Z,11(13)-trien-6,12-olide (18), and 8beta-tigloyloxy-3beta,14-dihydroxy-6betaH,7alphaH-germacra-1(10)Z,4E,11(13)-trien-6,12-olide (19), were isolated from the whole plant of Eupatorium lindleyanum. Eupalinilide B (2), Eupalinilide C (3), Eupalinilide E (5), Eupalinilide F (6), and Eupalinilide I (9) have been tested for cytotoxicity against P-388 and A-549 tumor cell lines.
CONCLUSIONS:
The results showed that eupalinilide B (2) and Eupalinilide E (5) demonstrated potent cytotoxicity. The structures of these compounds were determined by spectroscopic methods including 1D and 2D NMR spectra. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)