| In vivo: |
| Sci Transl Med. 2015 May 20;7(288):288ra77. | | The cytoplasmic prolyl-tRNA synthetase of the malaria parasite is a dual-stage target of febrifugine and its analogs.[Pubmed: 25995223] | The emergence of drug resistance is a major limitation of current antimalarials. The discovery of new druggable targets and pathways including those that are critical for multiple life cycle stages of the malaria parasite is a major goal for developing next-generation antimalarial drugs.
METHODS AND RESULTS:
Using an integrated chemogenomics approach that combined drug resistance selection, whole-genome sequencing, and an orthogonal yeast model, we demonstrate that the cytoplasmic prolyl-tRNA (transfer RNA) synthetase (PfcPRS) of the malaria parasite Plasmodium falciparum is a biochemical and functional target of Febrifugine and its synthetic derivative halofuginone. Febrifugine is the active principle of a traditional Chinese herbal remedy for malaria. We show that treatment with Febrifugine derivatives activated the amino acid starvation response in both P. falciparum and a transgenic yeast strain expressing PfcPRS. We further demonstrate in the Plasmodium berghei mouse model of malaria that halofuginol, a new halofuginone analog that we developed, is active against both liver and asexual blood stages of the malaria parasite.
CONCLUSIONS:
Halofuginol, unlike halofuginone and Febrifugine, is well tolerated at efficacious doses and represents a promising lead for the development of dual-stage next-generation antimalarials. | | Southeast Asian J Trop Med Public Health. 2008 Nov;39(6):949-58. | | Possible involvement of IFN-gamma in early mortality of Plasmodium berghei NK65-infected BALB/c mice after febrifugine treatment.[Pubmed: 19062681] |
METHODS AND RESULTS:
Parasitemia patterns, survival and cytokine levels of Plasmodium berghei NK65-infected BALB/c mice, treated orally with the alkaloidal mixture of Febrifugine and isoFebrifugine at a dose of 1 mg/kg twice a day for 4 consecutive days were monitored. Whereas the untreated mice showed a progressive increase in parasitemia and ultimate death, the alkaloid mixture-treated group showed a transient suppression of parasitemia during the course of treatment. However, the parasitemia increased on discontinuation of treatment, leading to earlier death of mice in the treated group than in the infected but untreated controls. Mice in the infected but untreated group displayed a significant elevation in serum IFN-gammay levels during the first week post-infection (pI) and from Day 14 pI, relative to the levels in the uninfected controls. In contrast, although mice in the alkaloid mixture-treated group displayed no significant elevation in serum IFN-gamma levels during the first week pI, they showed considerable levels on Day 14 pI. There were no significant differences in serum IL-4 levels among the groups. The titers of the parasite-specific IgG1, IgG2a, IgG2b and IgG3 were significantly elevated from Day 11 pI in both the treated and untreated groups. There was a significant difference in survival duration between the IFN-gamma-/- mutant and BALB/c mice. IFN-gamma-/- mutant mice showed a decrease in parasitemia levels while receiving medication, which was significantly lower than those of the treated BALB/c mice.
CONCLUSIONS:
The results of the present study suggest that although IFN-gamma is significant for protective immunity in mice with malaria infection, it may play an adverse role post-medication, causing earlier mortality of treated BALB/c mice. |
|


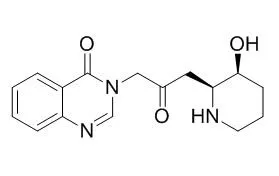



 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)