| Structure Identification: |
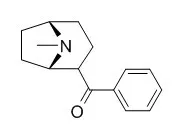
| Tetrahedron,2012,68(39): 8236–8244. | | Approaches to the enantioselective synthesis of ferrugine and its analogues[Reference: WebLink] | A four-step synthetic route, to Ferrugine (2α-benzoyltropane), its methyl analogue (2-acetyltropane) and their N-benzyl analogues is reported.
METHODS AND RESULTS:
The reaction sequence uses tropinone or N-benzylnortropinone aldols as key intermediates. Reduction of aldol derived N-tosylhydrazones and oxidation of the side chain hydroxyl group followed by spontaneous diastereomer equilibration provides the final products. Relative configuration of the exo,anti N-methyl and N-benzyl aldols was retained during N-tosylhydrazone formation. The relative stereochemistry of N-tosylhydrazones was assigned by single crystal diffraction.
CONCLUSIONS:
The final products, Ferrugine and its methyl analogue, were synthesized in enantiomerically pure form via asymmetric deprotonation of tropinone using chiral lithium amide/lithium chloride aggregate prepared in situ from (S,S)-N,N-bis(1-phenylethyl)amine hydrochloride. | | Tetrahedron Letters,2009,50(51):7196–7198. | | Enantioselective route to ferrugine and its methyl analogue via aldol deoxygenation[Reference: WebLink] | A simple enantioselective approach to Ferrugine (2α-benzoyltropane) and its methyl analogue (2-acetyltropane) is reported.
METHODS AND RESULTS:
The four-step sequence uses an enantioselective aldol reaction of tropinone with benzaldehyde or acetaldehyde, combined with an aldol deoxygenation via tosylhydrazone reduction and oxidation of the side-chain hydroxy group. The final products, Ferrugine and its methyl analogue, are prepared in 35% and 23% overall yields, respectively.
CONCLUSIONS:
Both enantiomers of the products (ee 90–99%) are accessible via the same route using either enantiomer of N,N-bis(1-phenylethyl)amine hydrochloride as the chiral reagent. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)