| In vitro: |
| Steroids. 2014 May;83:45-51. | | Longipetalosides A-C, new steroidal saponins from Tribulus longipetalus.[Pubmed: 24530871 ] |
METHODS AND RESULTS:
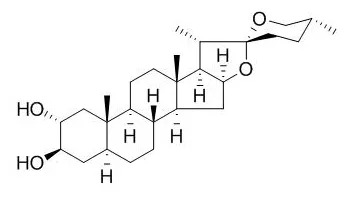
Longipetalosides A-C (1-3); three new furostane steroidal saponins together with (25S)-5α-furastan-3β,22,26-triol (4) and Gitogenin (5) were isolated from the methanolic extract of the whole plant of Tribulus longipetalus.
The structures of these compounds (1-5) were established by using 1D ((1)H, (13)C) and 2D NMR (HMQC, HMBC, COSY, NOESY) spectroscopy, and mass spectrometry (ESIMS, HRESIMS), and in comparison with literature data reported for related compounds. Compounds 1-5 were evaluated for their inhibitory activities against enzymes α-glucosidase, lipoxygenase, acetylcholinesterase, and butyrylcholinesterase.
CONCLUSIONS:
Only the compounds 4 and 5 were found as the inhibitors of enzyme α-glucosidase with IC50 values of 33.5±0.22 and 37.2±0.18μM, respectively. | | Pharmacol Res. 2016 Aug;110:139-150. | | Drug interaction study of natural steroids from herbs specifically toward human UDP-glucuronosyltransferase (UGT) 1A4 and their quantitative structure activity relationship (QSAR) analysis for prediction.[Pubmed: 27208893 ] | The wide application of herbal medicines and foods containing steroids has resulted in the high risk of herb-drug interactions (HDIs).
The present study aims to evaluate the inhibition potential of 43 natural steroids from herb medicines toward human UDP- glucuronosyltransferases (UGTs).
METHODS AND RESULTS:
A remarkable structure-dependent inhibition toward UGT1A4 was observed in vitro. Some natural steroids such as Gitogenin, tigogenin, and solasodine were found to be the novel selective inhibitors of UGT1A4, and did not inhibit the activities of major human CYP isoforms. To clarify the possibility of the in vivo interaction of common steroids and clinical drugs, the kinetic inhibition type and related kinetic parameters (Ki) were measured. The target compounds 2-6 and 15, competitively inhibited the UGT1A4-catalyzed trifluoperazine glucuronidation reaction, with Ki values of 0.6, 0.18, 1.1, 0.7, 0.8, and 12.3μM, respectively. And this inhibition of steroids towards UGT1A4 was also verified in human primary hepatocytes. Furthermore, a quantitative structure-activity relationship (QSAR) of steroids with inhibitory effects toward human UGT1A4 isoform was established using the computational methods.
CONCLUSIONS:
Our findings elucidate the potential for in vivo HDI effects of steroids in herbal medicine and foods, with the clinical dr ugs eliminated by UGT1A4, and reveal the vital pharamcophoric requirement of natural steroids for UGT1A4 inhibition activity. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)