| In vitro: |
| Jpn J Pharmacol. 1993 Apr;61(4):351-6. | | Non-competitive antagonism by hirsuteine of nicotinic receptor-mediated dopamine release from rat pheochromocytoma cells.[Pubmed: 8320880] | Effects of Hirsuteine, an indole alkaloid extracted from Uncaria genus, on nicotine- and high K-induced responses were investigated in rat pheochromocytoma PC12 cells.
METHODS AND RESULTS:
Hirsuteine (300 nM-10 microM) inhibited dopamine release evoked by 100 microM nicotine in a concentration-dependent manner. Hirsuteine did not produce a parallel shift of the concentration-response relationship curve for nicotine, but reduced maximal dopamine release. Dopamine release evoked by 60 and 155 mM KCl was also inhibited by Hirsuteine, but the concentration necessary for significant inhibition was higher (more than 10 microM). Under whole cell voltage-clamp, Hirsuteine reversibly inhibited inward currents activated by 100 microM nicotine. The current inhibition was slightly accelerated by hyperpolarization.
CONCLUSIONS:
The results suggest that Hirsuteine non-competitively antagonizes nicotine-evoked dopamine release by blocking ion permeation through nicotinic receptor channel complexes. The blockade of Ca channels, which are activated during nicotine-evoked depolarization, may not play a major role in the antagonism. | | J Asian Nat Prod Res. 2014;16(8):876-83. | | Alkaloids from the hook-bearing branch of Uncariarhynchophylla and their neuroprotective effects against glutamate-induced HT22 cell death.[Pubmed: 24899363] |
METHODS AND RESULTS:
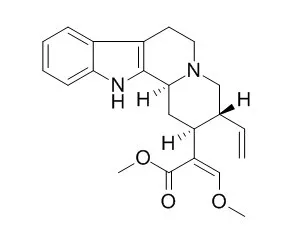
One new alkaloid, 4-geissoschizine N-oxide methyl ether (1), was isolated from the EtOH extract of the hook-bearing branch of Uncariarhynchophylla, together with 10 known alkaloids, 3-epi-geissoschizine methyl ether (2) isolated from U.rhynchophylla for the first time, geissoschizine methyl ether (3), 4-Hirsuteine N-oxide (4), Hirsuteine (5), hirsutine (6), 3α-dihydro-cadambine (7), 3β-isodihydro-cadambine (8), cadambine (9), strictosamide (10), and akuammigine (11). The structures were elucidated by spectroscopic methods including UV, ESI-QTOF MS, NMR, and circular dichroism experiments. Neuroprotective effects of 1-9 were investigated against 3 mM glutamate-induced HT22 cell death.
CONCLUSIONS:
The activity assay showed that 2, 3, 5, and 6 exhibited potent neuroprotective effects against glutamate-induced HT22 cell death. However, only weak neuroprotective activities were observed for 1, 4, 7, 8, and 9. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)