| In vitro: |
| Chemistry & Biodiversity,2006,3(2):224–230. | | New Antioxidant Phenolic Glycosides from Walsura yunnanensis[Reference: WebLink] |
Four new polyphenolic glycosides (1–4) were isolated from the BuOH extract of the bark of Walsura yunnanenis C. Y. Wu.
METHODS AND RESULTS:
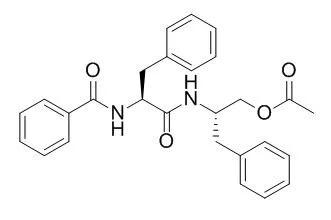
These compounds comprise two lignans, i.e., the 9-O-α-l-arabinopyranosides 1 and 2 of (−)-isolariciresinol and of (+)-5-methoxyisolariciresinol, respectively, and the two phenolic glycosides 3,4,5-trimethoxyphenyl 2-O-(α-l-fucopyranosyl)-β-d-glucopyranoside (3) and 3,5-dihydroxyphenyl 6-O-(4-hydroxy-3,5-dimethoxybenzoyl)-β-d-glucopyranoside (walsuraside; 4). In addition, three known compounds, 3,4,5-trimethoxyphenyl β-d-glucopyranoside, Asperglaucide, and butyl α-d-fructofuranoside were identified. Their structures were elucidated spectroscopically and by chemical transformation (hydrolysis).
CONCLUSIONS:
The new compounds 1, 2, and 4 displayed significant antioxidant activities, with IC50 values in the range of ca. 42 to 49 μg/ml. | | Quím Nova, 2003, 26(3):335-9 | | Isolamento e avaliação da atividade nematicida de constituintes químicos de Mucuna cinerea contra Meloidogyne incognita e Heterodera glycines[Reference: WebLink] |
METHODS AND RESULTS:
Phytochemical investigation of the aerial parts and roots of mucuna cinerea led to the isolation of a mixture of fatty acids, triacylglicerols, b-sitosterol, stigmasterol, stigmasterol glucoside, daucosterol, Asperglaucide (4) and the isoflavonoids prunetin (1), genistein (2), medicarpin (3), daidzein (5), 7-o-a-glycopiranosyl daidzein (6). an in vitro bioassay was carried out with compounds 1-4, at the concentration of 50 and 5 mg ml-1 against the phytonematodes m. incognita and h. glycines.
CONCLUSIONS:
although the four compounds showed some nematocidal property, the most active was (1), causing 70% mortality of m. incognita at the concentration of 50 mg ml-1. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)