| Structure Identification: |
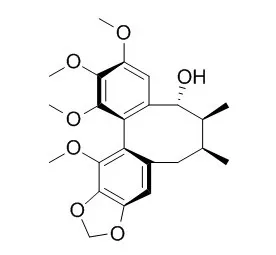
| Chemical & pharmaceutical bulletin 27(11), 2695-2709, 1979-11-25 | | The Constituents of Schizandra chinensis BAILL. V. The Structures of Four New Lignans, Gomisin N, Gomisin O, Epigomisin O and Gomisin E, and Transformation of Gomisin N to Deangeloylgomisin B[Reference: WebLink] | Four new dibenzocyclooctadiene lignans, gomisin N (1), Gomisin O (2) and gomisin E (4), and epiGomisin O (3), together with a known lignan (+)-deoxyschizandrin (5) were isolated from the fruits of Schizandra chinensis BAILL. (Schizandraceae).
METHODS AND RESULTS:
The structures of the new lignans were elucidated by chemical and spectral studies. The transformation of 1 to deangeloylgomisin B (debenzoylgomisin C) (6) is also described. | | J Org Chem. 2005 Oct 28;70(22):8932-41. | | Asymmetric total synthesis of dibenzocyclooctadiene lignan natural products.[Pubmed: 16238330 ] | Full details of the asymmetric total syntheses of the dibenzocyclooctadiene lignans interiotherin A, angeloylgomisin R, Gomisin O, and gomisin E (epiGomisin O) are presented.
METHODS AND RESULTS:
The syntheses were based on a unified synthetic strategy involving a novel crotylation using the Leighton auxiliary that occurred with excellent asymmetric induction (>98:2 enantiomeric ratio), a diastereoselective hydroboration/Suzuki-Miyaura coupling reaction sequence, and an atropdiastereoselective biarylcuprate coupling, both of which occurred with total (>20:1) stereocontrol.
The syntheses were achieved in six to eight steps from simple aromatic precursors. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)