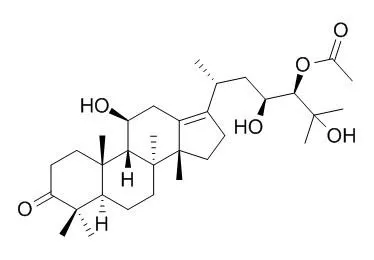
| In vitro: |
| Anal Bioanal Chem. 2011 Jan;399(3):1363-9. | | A sensitive liquid chromatography-mass spectrometry method for simultaneous determination of alisol A and alisol A 24-acetate from Alisma orientale (Sam.) Juz. in rat plasma.[Pubmed: 21107819] |
METHODS AND RESULTS:
A liquid chromatography-mass spectrometry (LC-MS) method was developed and validated for the simultaneous determination of alisol A and Alisol A 24-acetate from Alisma orientale (Sam.) Juz. in rat plasma using diazepam as an internal standard.
A 200-μl plasma sample was extracted by methyl tert-butyl ether and the separation was performed on Kromasil C(18) column (150 × 4.6 mm, 5 μm) with the mobile phase of acetonitrile (containing 0.1% of formic acid)-water (73:27, v/v) at a flow rate of 0.8 ml/min in a run time of 10 min. The two analytes were monitored with positive electrospray ionization by selected ion monitoring mode. The lower limit of quantitation for both alisol A and Alisol A 24-acetate were 10 ng/ml. The calibration curves were linear in the measured range 10-1,000 ng/ml for alisol A and 10-500 ng/ml for Alisol A 24-acetate. The mean extraction recoveries were above 74.7% for alisol A and above 72.4% for Alisol A 24-acetate from biological matrixes. The intra- and inter-day precision for all concentrations of quality controls was lower than 14.1% (RSD %) for each analyte. The accuracy ranged from -12.3% to 9.8% (RE %) for alisol A, and -8.6% to 14.2% (RE %) for Alisol A 24-acetate.
CONCLUSIONS:
The method was successfully applied to the study on the pharmacokinetics of alisol A and Alisol A 24-acetate in rat plasma. | | Arch Pharm Res. 2003 Jun;26(6):463-5. | | Anti-complementary activity of protostane-type triterpenes from Alismatis rhizoma.[Pubmed: 12877555] | Four protostane-type triterpenes, alisol B 23-acetate (1a), alisol C 23-acetate (2a), alisol B (3a), and Alisol A 24-acetate (4a), were isolated from the rhizome of Alismatis plantago-aquatice L. var. orientale Samuelson (Alismataceae) and eleven protostane derivatives (compounds 1-11) were obtained by selective modification from alisol B 23-acetate (1a).
METHODS AND RESULTS:
These compounds were investigated for their anti-complement activity against the classical pathway of the complement system. Alisol B (3a) and Alisol A 24-acetate (4a) exhibited anti-complement activity with IC50 values of 150 and 130 microM. Among the synthetic derivatives, the tetrahydroxylated protostane triterpene (9) showed moderate inhibitory activity with IC50 value of 97.1 microM.
CONCLUSIONS:
Introduction of an aldehyde group at C-23 (10; IC50 value, 47.7 microM) showed the most potent inhibitory effect on the complement system in vitro. | | Arch Pharm Res. 2012 Nov;35(11):1919-26. | | A new triterpenoid from Alisma orientale and their antibacterial effect.[Pubmed: 23212633] | A new triterpenoid, named alisol Q 23-acetate, as well as fourteen known terpenes, alisol B 23-acetate (2), alisol B (3), alismol (4), 10-O-methyl-alismoxide (5), alismoxide (6), 11-deoxyalisol C (7), 13β,17β-epoxyalisol B 23-acetate (8), 4β,12-dihydroxyguaian-6,10-diene (9), alisol C 23-acetate (10), alisolide (11), 16β-methoxyalisol B monoacetate (12), alisol A (13), 16β-hydroxyalisol B 23-acetate (14), Alisol A 24-acetate (15) were isolated from the rhizomes of Alisma orientale.
The structures of compounds (1-15) were identified based on 1D and 2D NMR, including (1)H-(1)H COSY, HSQC, HMBC and NOESY spectroscopic analyses.
METHODS AND RESULTS:
Among these isolates, antibacterial effect of compounds 2, 3, 10, and 15, major constituents of A. orientale was examined. The MIC values of compounds 2, 10, and 15 were 5-10 βg/mL against eight antibiotic resistant strains, which were lower than those from the positive controls (MICs of chloramphenicol and ampicillin were 5-80 μg/mL).
CONCLUSIONS:
Therefore, compounds 2, 10 and 15 exhibited the potent antibacterial activity. |
|
| In vivo: |
| Molecules. 2016 Jan 9;21(1):74. | | The Protective Effects of Alisol A 24-Acetate from Alisma canaliculatum on Ovariectomy Induced Bone Loss in Vivo.[Pubmed: 26760992 ] | Alisma canaliculatum is a herb commonly used in traditional Korean medicine, and has been shown in scientific studies to have antitumor, diuretic hepatoprotective, and antibacterial effects.
Recently, the anti-osteoclastogenesis of Alisol A 24-acetate from Alisma canaliculatum was investigated in vitro. However, the influence of Alisol A 24-acetate on osteoporosis in animals has not been investigated.
METHODS AND RESULTS:
The present study was undertaken to investigate the anti-osteoporotic effect of Alisol A 24-acetate on bone mass in ovariectomized (OVX) mice and to identify the mechanism responsible for its effects. OVX mice were treated daily with 0.5 or 2 μg/g of Alisol A 24-acetate for a period of six weeks. It was found that these administrations significantly suppressed osteoporosis in OVX mice and improved bone morphometric parameters. The serum estradiol, bone alkaline phosphatase levels, regulatory T/Th17 cell numbers were significantly increased by Alisol A 24-acetate as compared with untreated OVX mice. In addition, TRAP activity was inhibited by Alisol A 24-acetate in OVX mice.
CONCLUSIONS:
These results suggest Alisol A 24-acetate effectively prevents bone loss in OVX mice, and that it can be considered a potential therapeutic for the treatment of postmenopausal osteoporosis. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)