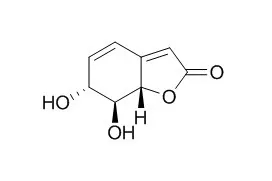
| Structure Identification: |
| Bioorg Med Chem. 2011 Sep 1;19(17):5225-30. | | Tylosema esculentum extractives and their bioactivity.[Pubmed: 21813280] |
METHODS AND RESULTS:
The investigation of Tylosema esculentum (Morama) husks, cotyledons, and tuber yielded Griffonilide 2, compound 1, griffonin 3, gallic acid 4, protocatechuic acid 5, β-sitosterol 6, behenic acid 7, oleic acid 8, sucrose 9, 2-O-ethyl-α-D-glucopyranoside 10, kaempferol 11 and kaempferol-3-O-β-D-glucopyranoside 12. The structures of the isolates were determined by NMR, HR-TOF EIMS, IR and UV-vis spectroscopy, and by comparison with literature data. The husk EtOAc and n-butanol extracts demonstrated >90% DPPH radical scavenging activity at concentrations of 25, 50 and 250 μg/mL. Furthermore the husk extracts showed higher total phenolic content (233 mg GAE/g). The extractives exhibited minimum inhibitory quantities of 50-100 μg or no activity against Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa and Candida albicans. The tuber extracts were inactive against Caco-2 and Hela cell lines, while the husk extracts showed low activity against Caco-2 and Vero cell line with IC(50) values >400 μg/mL.
CONCLUSIONS:
The GC-MS analysis showed the beans and tuber non-polar (n-hexane) extracts major constituents as fatty acids. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)